��Ŀ����
��������Ԫ��R�����������ﵽ����ʱ���������С�ڴ��������ڲ������֮����ҵ������ڲ�����������������������R����ȷ˵�� �� ��
�ٳ��������ȶ����ڵ�R�������ﶼ�������� ��R����̬�⻯�ﶼ�����ȷֽ⡣
��RԪ�ض���������̬��������Ȼ���� ��R������������Ӧˮ���ﶼ��ǿ�
��R���⻯���ڿ����ж���ȼ������+4��R��������
��R�ĵ��ʹ�̬ʱ����ͬһ���͵ľ��壻
��R�ĺ�������������ȶ�������c(H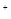 )��c(OH
)��c(OH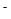 )
)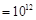 ����Һ�У�
����Һ�У�
�ٳ��������ȶ����ڵ�R�������ﶼ�������� ��R����̬�⻯�ﶼ�����ȷֽ⡣
��RԪ�ض���������̬��������Ȼ���� ��R������������Ӧˮ���ﶼ��ǿ�
��R���⻯���ڿ����ж���ȼ������+4��R��������
��R�ĵ��ʹ�̬ʱ����ͬһ���͵ľ��壻
��R�ĺ�������������ȶ�������c(H
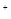 )��c(OH
)��c(OH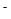 )
)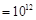 ����Һ�У�
����Һ�У�| A���٢ۢ� | B���٢ڢ� | C���٢ڢݢ� | D���ۢݢޢ� |
C
����������������ڴ��������ڲ������֮����8��2��6������Ϊ���������ﵽ����ʱ����������������ڲ��������������������˵��RӦ����S��Si��SO2�����������������趼������������ȷ��H2S��SiH4�������ֽ⣬����ȷ��������Ȼ����ȫ�����Ի���̬����ʽ���ڣ��۲���ȷ�����������ᣬ�ܲ���ȷ������ȷ������ȷ�����ʹ��γɵľ�����ԭ�Ӿ��壬S�����γɵ��Ƿ��Ӿ��壻����ˮ�����ӻ�������֪�������Һ��c(H
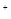 )��c(OH
)��c(OH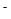 )
)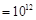 ������Һ��������Ũ����0.1mol/L������SO32����SiO32���������Ӳ��ܴ������棬��ѡC��
������Һ��������Ũ����0.1mol/L������SO32����SiO32���������Ӳ��ܴ������棬��ѡC�������������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ�������������ѧ����������������Ӧ�������������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

��ϰ��ϵ�д�
�����Ŀ
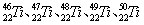 �ȡ���Щͬλ��ԭ�ӵ�������������Ϊ
�ȡ���Щͬλ��ԭ�ӵ�������������Ϊ