��Ŀ����
6��ͭ�����������������϶�Ľ�������1�������й�ͭԪ�ص�˵���У�����ȷ����BD��
A����ͭ������֡�Ӳ�����ǺϽ�
B��ͭ�������γ����ܵ�����Ĥ
C��ͭ��O2��Ӧ�����ɺ�ɫ��CuO
D��CuSO4•5H2O��һ�ֻ������Ⱥ��Ϊ��ɫ�Ĺ���
��2��ij��ѧС��Ϊ�ⶨһ��������ijͭ���������ͭ���������������������ʵ�鷽����
������ͭ�������$��_{��ַ�Ӧ}^{������ҺA}$�ⶨ������������
������ͭ�������$��_{��ַ�Ӧ}^{������ҺB}$�ⶨʣ����������
����ҺA�ܷ�ѡ��ϡ������ܣ�д���������з�����Ӧ�����ӷ���ʽ��������˵������3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��Al+4H++NO3-=Al3++NO��+2H2O������������ҺA��ϡHNO3����������NO���壬��֪ͭ������������������������ͨ���з���������ͭ������������
����ҺB��ѡ��ϡ���ᣬҲ��ѡ������������Һ��
��ʵ�����з��������������ʵʩ��
���� ��1��A�����ݺϽ�Ķ����жϣ�
B��ͭ���ɼ�ʽ̼��ͭ����Ϊ���ɣ�
C��ͭ��O2��Ӧ��������ͭ��Ϊ��ɫ���壻
D��CuSO4•5H2OΪ�����
��2����Ϊ���ý���������ǿ�ᣬҲ����ǿ�Ӧ�������������������������֪����������������ȷ��ͭ������������Ҳ����ϡ���ᷴӦ����������NO���������ϻ������������з��������ͭ������������
��� �⣺��1��A����ͭ��ͭ���Ͻ𡢲���������������Ͻ�Ӳ�������衢þ���γɵĺϽ𣬹�A��ȷ��
B��ͭ���ɼ�ʽ̼��ͭ����Ϊ���ɣ���B����
C��ͭ��O2��Ӧ��������ͭ��Ϊ��ɫ���壬��C��ȷ��
D��CuSO4•5H2OΪ��������Ⱥ��Ϊ��ɫ�Ĺ���CuSO4����D����
�ʴ�Ϊ��BD��
��2���ټ���ϡ���ᣬ�ɷֱ���3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��Al+4H++NO3-=Al3++NO��+2H2O����������NO����������ͭ������������������������ͨ���з���������ͭ������������
�ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��Al+4H++NO3-=Al3++NO��+2H2O������������ҺA��ϡHNO3����������NO���壬��֪ͭ������������������������ͨ���з���������ͭ������������
������Ϊ���ý���������ǿ�ᣬҲ����ǿ�Ӧ������������Ҳ��������������Һ��Ӧ���ʴ�Ϊ������������Һ��
��ʵ�������NO���ױ��������ɶ������������Բ�������ʵ��������������ɸ������������ȷ����������������ȷ��ͭ�������������ʴ�Ϊ����
���� �����ۺϿ������ʵĺ����IJⶨ��Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬ע�����ͭ�����������Լ�ʵ���ԭ�������ۣ��ѶȲ���

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�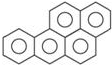 ����[��]����һ�ֳ��������������ķ������о��°�ЧӦ�IJ��ձ�������ṹʽ����5�������ಢ�ϣ���ͼ��ÿ��̼ԭ���϶���һ��˫����C=C���������й���m��˫���������������ͬһ��ƽ���ϣ������п���ͬʱ����ͬһ��ƽ���ϵ�ԭ����Ϊn������m��n�ֱ���ڣ�������
����[��]����һ�ֳ��������������ķ������о��°�ЧӦ�IJ��ձ�������ṹʽ����5�������ಢ�ϣ���ͼ��ÿ��̼ԭ���϶���һ��˫����C=C���������й���m��˫���������������ͬһ��ƽ���ϣ������п���ͬʱ����ͬһ��ƽ���ϵ�ԭ����Ϊn������m��n�ֱ���ڣ�������| A�� | 10��30 | B�� | 10��32 | C�� | 11��30 | D�� | 11��32 |
| A�� | ��������ƽ����5.22gʳ�� | |
| B�� | ��100mL����Ͳȡ5.2mLϡ���� | |
| C�� | ��100mL������ƿ����50mL 0.1mol/L���� | |
| D�� | ��25mL����ʽ�ζ�����ȡ15.12mL���������Һ |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |
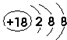 ��
����2����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����KOH��
��3���ݵĵ���������������Һ��Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2�����ݵ���������������������Һ��Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
��4�����ߵ���ͨ��������ɵĻ������У�������Ӧ�����ӷ���ʽ��Cl2+2Br-=2Cl-+Br2��
| A�� | Na2O2��Na2O | B�� | KClO��NaOH | C�� | Br2��HBr | D�� | HF��H2O |
| ���� | NaCl | MgO | AlBr3 | SiCl4 | ���ۻ�����R |
| �۵㣨�棩 | 801 | 2852 | 97.5 | -70 | 1723 |
| �е㣨�棩 | 1413 | 3600 | 263.3 | 57 | 2230 |
| A�� | SiCl4�Ƿ��Ӿ��� | B�� | MgO��NaCl�ľ����ܴ� | ||
| C�� | R��ԭ�Ӿ��� | D�� | AlBr3Ϊ���Ӿ��� |
| A�� | �Ҵ��������dz��õ�ζƷ����Ҫ�ɷ� | |
| B�� | 75%��������������Ҵ���Һ������ҽ������ | |
| C�� | ά����C���л�ԭ�ԣ������������������� | |
| D�� | ����������ľ�������ά�����ά�����������ǽ������� |
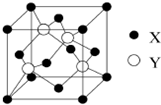 Ԫ��Xλ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ1��Ԫ��Y��̬ԭ�ӵ�3p�������5�����ӣ�Ԫ��Z��ԭ�����������������ڲ��3����Ԫ��W��̬ԭ�Ӻ�����ӹ���16���˶�״̬��
Ԫ��Xλ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ1��Ԫ��Y��̬ԭ�ӵ�3p�������5�����ӣ�Ԫ��Z��ԭ�����������������ڲ��3����Ԫ��W��̬ԭ�Ӻ�����ӹ���16���˶�״̬��