��Ŀ����
����Ŀ��ʵ����������ͼװ�ý����к��ȵIJⶨ���ش��������⣺
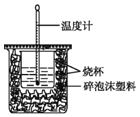
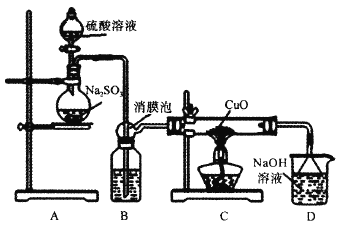
(1)��ͼ����һ��δ����������_______����������)��
(2)�ڲ�����ȷ��ǰ��������к��Ȳⶨ��ȷ�ԵĹؼ���____��
(3)�����0.50 mol/L��������������ƹ������ʵ�飬����ݴ�ʵ���������д�к��ȵ��Ȼ�ѧ����ʽ�е�H��____(����ƫ������ƫС������������)��ԭ����_______��
(4)����50 mL 0.50 mol/L��ϡ������50 mL 0.55 mol/L��ϡ����������Һ����ʵ�飬����Һ���ܶȾ�Ϊ1 g/cm3���кͺ���Һ�ı�����c=4.18 J/(g����)�������ʵ�����ݼ����к���H= __ (ȡС�����һλ)��
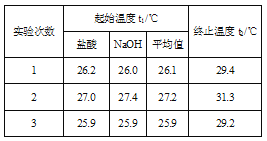
(5)����(4)�еĽ�����к��ȵ�����ֵ��ƫ�����ƫ���ԭ�������___��
a.ʵ��װ�ñ��¡�����Ч����
b.����ȡNaOH��Һ�����ʱ���Ӷ���
c.�ֶ�ΰ�NaOH��Һ����ʢ��ϡ�����С�ձ���
d.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������Һ���¶�
���𰸡����β�������� ��������ɢʧ ƫС NaOH�����ܽ���� -55.2 kJ��mol-1 acd
��������
(1)�ⶨ�к��ȣ�Ҫ�û��β�����������裻
(2)�к��Ȳⶨ��ȷ�ⶨ�����仯��
(3)�������ƹ��ܽ���ȣ�
(4)���ݹ�ʽQ=cm��T���������0.025mol��ˮ�ų��������������к��ȵĸ����������1molˮ�ų���������
��5��55.2С���к���57.3��˵��ʵ���������������ʧ��
(1) �ⶨ�к��ȣ�Ҫ�û��β�����������裬��ͼδ�������β����������
(2)�к��Ȳⶨ��ȷ�ⶨ�����仯������к��Ȳⶨ��ȷ�ԵĹؼ��Ǽ�������ɢʧ��
(3) NaOH�����ܽ���ȣ������0.50 mol/L��������������ƹ������ʵ�飬�����ƫ�࣬���ݴ�ʵ���������д�к��ȵ��Ȼ�ѧ����ʽ�е�H��ƫС��
(4) 3�η�Ӧǰ���¶Ȳ�ֱ�Ϊ��3.3����4.1����3.3�����ڶ�����ȥ��ƽ��ֵΪ3.3����50 mL 0.50 mol/L��ϡ������50 mL 0.55 mol/L��ϡ����������Һ��������m=100mL��1g/mL=100g��c=4.18J/��g���������빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18J/��g������100g��3.3��=1.3794kJ��������0.025mol��ˮ�ų�����1.3794kJ����������1mol��ˮ�ų�����Ϊ1.3794kJ��40=55.2kJ������ʵ���õ��к�����H=-55.2kJ/mol��
��5��a��װ�ñ��¡�����Ч���������ʧ��õ�����ƫС���к��ȵ���ֵƫС����ѡa��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ�ʲ�ѡb��
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�������ʧ��õ�����ƫС���к��ȵ���ֵƫС����ѡc��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨHCl��Һ���¶ȣ�HCl��Һ����ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС����ѡd����Ϊacd��

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�