��Ŀ����
��16�֣��Ų��������Ļ�ѧ��������������Fe2O3�������ǵ��ӡ����Ź�ҵ�Ĵ��Բ��ϣ���ҵ�ϲ��������Ѱ۵��½��ϣ�������FeSO4�ķ�Һ��Ϊԭ�����Ʊ��Ų���������
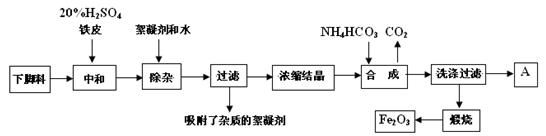

��֪�����еĻ�ѧ��Ӧ����ʽΪ��FeCO3 �� FeO + CO2����4FeO + O2 �� 2Fe2O3
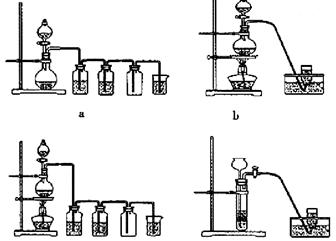
��1����98%��H2SO4������500mL��20%��H2SO4�����貣��������
E��500mL����ƿ F����ͷ�ι�
��2��Ũ���ᾧ��õ��ľ����� ���ѧʽ����A�������� ��
����Һ�и����ӵ�Ũ�ȱȽϴ�СΪ�� ��
��3��20%H2SO4����Ƥ�����÷ֱ��� ��
��4��������Һ�к���NH4+�ķ�����
��5��д�����衰�ϳɡ��з����Ļ�ѧ�仯���û�ѧ����ʽ��ʾ����
��


��֪�����еĻ�ѧ��Ӧ����ʽΪ��FeCO3 �� FeO + CO2����4FeO + O2 �� 2Fe2O3
��1����98%��H2SO4������500mL��20%��H2SO4�����貣��������
| A�������� | B���ձ� | C��©�� | D��250mL����ƿ |
��2��Ũ���ᾧ��õ��ľ����� ���ѧʽ����A�������� ��
����Һ�и����ӵ�Ũ�ȱȽϴ�СΪ�� ��
��3��20%H2SO4����Ƥ�����÷ֱ��� ��
��4��������Һ�к���NH4+�ķ�����
��5��д�����衰�ϳɡ��з����Ļ�ѧ�仯���û�ѧ����ʽ��ʾ����
��
��16�֣�
��1��ABEF��2�֣���2����1�֣��д������֣�
��2��FeSO4��7H2O��2�֣���( NH4)2 SO4��2�֣���c(SO42-)>c(Fe2+)>c(H+)>c(OH-) ��3�֣�
��3����������Fe2+���Ƶ�ˮ�⣬��Ƥ�����÷�ֹFe2+����������2�֣���1�֣�
��4�����Թ���ȡ��Һ����������������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���飬����ֽ����ɫ����֤����Һ�к���NH4+����2�֣�
��5��FeSO4+2NH4HCO3 ="===" FeCO3��+( NH4)2 SO4+ CO2��+H2O��3�֣�
��1��ABEF��2�֣���2����1�֣��д������֣�
��2��FeSO4��7H2O��2�֣���( NH4)2 SO4��2�֣���c(SO42-)>c(Fe2+)>c(H+)>c(OH-) ��3�֣�
��3����������Fe2+���Ƶ�ˮ�⣬��Ƥ�����÷�ֹFe2+����������2�֣���1�֣�
��4�����Թ���ȡ��Һ����������������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���飬����ֽ����ɫ����֤����Һ�к���NH4+����2�֣�
��5��FeSO4+2NH4HCO3 ="===" FeCO3��+( NH4)2 SO4+ CO2��+H2O��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ

 ��ʾ��ͼ�ڷ����ڻ�����ϩ�ķ���װ��(�г�װ�ò��ػ�������Ҫ���ȵ������·��á����)��
��ʾ��ͼ�ڷ����ڻ�����ϩ�ķ���װ��(�г�װ�ò��ػ�������Ҫ���ȵ������·��á����)�� ��������̣�ʵ��ʱ��ҪѸ��Ϩ����棬�����ȫ�IJ��������ǣ� ��
��������̣�ʵ��ʱ��ҪѸ��Ϩ����棬�����ȫ�IJ��������ǣ� ��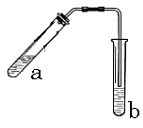
 ��ʵ���������������Ļ�ѧ����ʽ(Ҫ�����-18ʾ��ԭ��)
��ʵ���������������Ļ�ѧ����ʽ(Ҫ�����-18ʾ��ԭ��)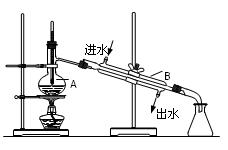
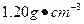 )����100 mL 0.200
)����100 mL 0.200 ����ʱ��������ʽ�ζ�����ȡ24.5������_______ mL,ϴ������ʽ�ζ���Ӧ�� ����������ȡ��������ᡣ
����ʱ��������ʽ�ζ�����ȡ24.5������_______ mL,ϴ������ʽ�ζ���Ӧ�� ����������ȡ��������ᡣ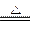 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O