��Ŀ����
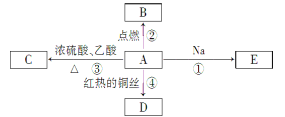
����Ŀ��ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С�

��1��д��A�з�Ӧ�Ļ�ѧ����ʽ______________________��
��2��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����________________��д���йصĻ�ѧ����ʽ__________________________________________________��
��3��C��ʢ��CCl4��������__________________��
��4����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���________��������______________��
���𰸡���1��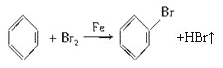
��2����ȥ�����屽�е��� Br2+2NaOH=NaBr+NaBrO+H2O
��3����ȥ�廯�������е���������4��ʯ����Һ ��Һ���ɫ(�������������)
�������������������1��A�з������������Ӧ����Ӧ�Ļ�ѧ����ʽΪ ��
��
��2��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ���dz�ȥ�����屽�е��壬��Ӧ�ķ���ʽ��Br2+2NaOH=NaBr+NaBrO+H2O��
��3�����ڵ������������л��ܼ�CCl 4�ܽ⣬HBr���ܣ�������C��ʢ��CCl 4�������dz�ȥ�廯�������е�����������ֹ����HBr�ļ��飻
��4����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤��������HBr��ˮ��Һ�����ԣ������һ����֤�ķ��������Թ�D�м���ʯ����Һ����Һ���ɫ�Ϳ���֤����

 ������ϵ�д�
������ϵ�д�����Ŀ�����ࡢ��֬��������������������������Ӫ�����ʡ�
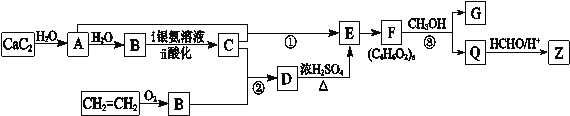
��1��Ϊ̽�����ǵ�ijЩ��ѧ���ʣ�ij�о���ѧϰС���ͬѧ���������ʵ�鷽�����±������ǽ���ʵ�����д��ʵ�鱨���еIJ������ݡ�
���� | ʵ�鲽�� | ʵ������ |
A | ��һ֧�ྻ���Թ��м���2mL20%��������Һ������ˮԡ�м���5min��Ȼ�����������Һ����ˮԡ�м��� | �����Ա仯 |
B | ��һ֧�ྻ���Թ��м���2mL20%������Һ������3��ϡ��������ˮԡ�м���5min��Ȼ���������������ͭ����Һ������������ | ��ש��ɫ�������� |
C | ��һ֧�ྻ���Թ��м���2mL20%������Һ������3��ϡ��������ˮԡ�м���5min��Ȼ�������������������Һ����Һ�ʼ��ԣ��ټ�������������ͭ����Һ������������ | ��ש��ɫ�������� |
��A��B�����������������ԭ��ֱ���________________________________________________��
��ȷ��ij����Һ�����Ƕ����������ǵķ�����________________________________________________��
��2����ͬ��֬�����Ժͼ���������ˮ��Ĺ�ͬ������________����֬��________����ᡱ�����������ˮ������ס�
��3����ʵ���У���ʱ���ϲ���մ������Ũ���ᣬ������մ������IJ�λ���ܳ��ֵ�����������________��ԭ����________________________________________________��