��Ŀ����
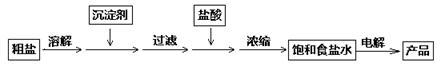
�����аѵ�ⱥ��ʳ��ˮ�Ĺ�ҵ�������ȼҵ���������������£�
��ȥʳ��ˮ�е�Ca2+��Mg2+��SO42����Ӧ�ֱ�������г���������NaOH��aq����
��Na2CO3(aq)����BaCl2��aq���������������˳��Ϊ ������ţ����������������Ϊ ��
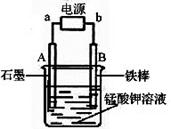
��2����ͼΪʵ���ҵ�ⱥ��ʳ��ˮ��ʵ��װ�á�X��Y��Ϊʯī�缫��������Χ��Һ�����м��η�̪��Һ��
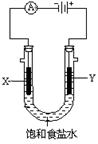
�ٵ��һ��ʱ���X�缫��������Һ�ܹ۲쵽�������� ��
Y�缫�ϵĵ缫��ӦʽΪ ������ü�����������ķ����� ��
�ڵ�ⱥ��ʳ��ˮ�����ӷ���ʽΪ ��������ʳ��ˮ���Ϊ200mL���Һ��Ե������е�����仯�������ܽ⣬���������ռ������壨�����֣�44.8mL����״����ʱ����Һ��pH= ��

��ȥʳ��ˮ�е�Ca2+��Mg2+��SO42����Ӧ�ֱ�������г���������NaOH��aq����
��Na2CO3(aq)����BaCl2��aq���������������˳��Ϊ ������ţ����������������Ϊ ��
��2����ͼΪʵ���ҵ�ⱥ��ʳ��ˮ��ʵ��װ�á�X��Y��Ϊʯī�缫��������Χ��Һ�����м��η�̪��Һ��

�ٵ��һ��ʱ���X�缫��������Һ�ܹ۲쵽�������� ��
Y�缫�ϵĵ缫��ӦʽΪ ������ü�����������ķ����� ��
�ڵ�ⱥ��ʳ��ˮ�����ӷ���ʽΪ ��������ʳ��ˮ���Ϊ200mL���Һ��Ե������е�����仯�������ܽ⣬���������ռ������壨�����֣�44.8mL����״����ʱ����Һ��pH= ��
��1���٢ۢڻ�ۢ٢ڻ�ۢڢ� ��ȥ������OH����CO32������NaOH��Na2CO3��
��2���� X�缫�������ݲ�����������Һ���ɫ����1�֣� 2Cl����2e��=Cl2��
��ʪ��ĵ���KI��ֽ���飬����ֽ������֤���ü�����Cl2�������𰸾����֣�
��2Cl����2H2O
 H2����Cl2����2OH�� 12
H2����Cl2����2OH�� 12�����������1�������Na2CO3��NaOH��BaCl2�ֱ��ȥCa2+��Mg2+��SO42����Ϊ�˳�ȥ�ɾ����Լ�����������������Ba2+�ú�����Na2CO3�����Լ����Լ���˳��ֻ��Ҫ��Na2CO3����BaCl2���漴�ɣ����������ȥ�����Na2CO3��NaOH����2����ⱥ��ʳ��ˮ������ӦΪ2H++2e��==H2����������ӦʽΪ2Cl����2e��=Cl2�����ܷ���ʽΪ2Cl����2H2O
 H2����Cl2����2��
H2����Cl2����2��n(H2)=n(Cl2)=44.8��22.4��10-3��2=10-3mol��n(OH��)=2n(H2)=2��10-3mol
c(OH��)=2��10-3��0.2=10-2��pH=12

��ϰ��ϵ�д�
�����Ŀ

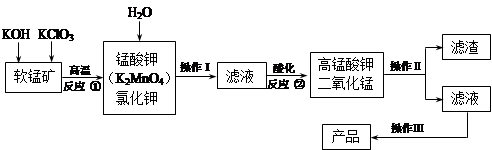

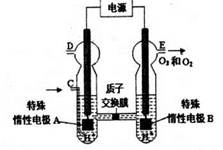
 2O3
2O3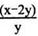 (����O3�ķֽ�)
(����O3�ķֽ�)