��Ŀ����
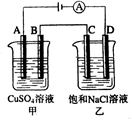
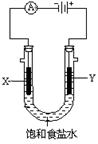
����O3�dz�����������ʵ���ҿ��õ��ϡ�����Ʊ���װ������ͼ����֪��Һ�зŵ�˳��O2>H+)������˵����ȷ����
A����C��ͨ��O2�����ʱ��Һ�е�������A����B��Ǩ��
B����C��ͨ��O2��A���ĵ缫��ӦʽΪ:2H++2e��=H2��
C����C����ͨ��O2�����Ʊ������ܷ�Ӧ�Ļ�ѧ����ʽ��3O2 2O3
2O3
D����C����ͨ��O2,D��E���ֱ��ռ���xL����yL ����(��״��)����E���ռ���yL������03��ռ���������Ϊ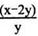 (����O3�ķֽ�)
(����O3�ķֽ�)

A����C��ͨ��O2�����ʱ��Һ�е�������A����B��Ǩ��
B����C��ͨ��O2��A���ĵ缫��ӦʽΪ:2H++2e��=H2��
C����C����ͨ��O2�����Ʊ������ܷ�Ӧ�Ļ�ѧ����ʽ��3O2
 2O3
2O3D����C����ͨ��O2,D��E���ֱ��ռ���xL����yL ����(��״��)����E���ռ���yL������03��ռ���������Ϊ
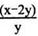 (����O3�ķֽ�)
(����O3�ķֽ�)D
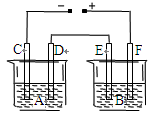
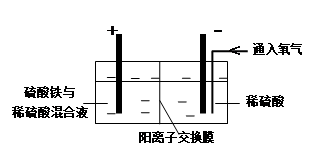
������������ϡ�����Ƴ���������Ӧ���������ɡ�
A��BΪ������AΪ���������ʱ����������Ǩ�ƣ�����
B��A��Ϊ����������Һ�зŵ�����02>H+������A����ӦΪO2+4H++4e-=2H2O������
C����C����ͨ���������Ʊ��ܷ�Ӧ����ʽΪ3H2O
 3H2��+O3��������
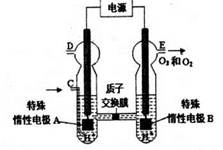
3H2��+O3��������D��D���缫��ӦΪ2H++2e-=H2����E���缫��ӦΪ2H2O-4e-=O2��+4H+��3H2O-6e-=O3��+6H+�������ɵ������ͳ����ֱ�Ϊamol��bmol����
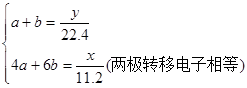 ����ã�b=
����ã�b=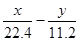 ����ȷ��
����ȷ��
��ϰ��ϵ�д�
 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ
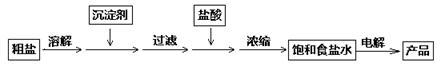
 R2Cu���л��ࣩ+ 2H����ˮ�ࣩ
R2Cu���л��ࣩ+ 2H����ˮ�ࣩ