��Ŀ����
6�� ԭ�������������������Ԫ��A��B��C��D��E������A�����ڱ���ԭ�Ӱ뾶��СԪ�أ�Bԭ�ӻ�̬ʱ���������������ڲ��������2����Dԭ�ӻ�̬ʱ2pԭ�ӹ������2��δ�ɶԵĵ��ӣ�E��ԭ������Ϊ29��
ԭ�������������������Ԫ��A��B��C��D��E������A�����ڱ���ԭ�Ӱ뾶��СԪ�أ�Bԭ�ӻ�̬ʱ���������������ڲ��������2����Dԭ�ӻ�̬ʱ2pԭ�ӹ������2��δ�ɶԵĵ��ӣ�E��ԭ������Ϊ29���ش��������⣨����Ԫ�ؾ���Ԫ�ط��ű�ʾ����
��1��B2A2������Bԭ�ӹ�����ӻ�����Ϊsp��1mol B2A2���ЦҼ�����ĿΪ3��6.02��1023��
��2��B��C��D��һ�������ɴ�СΪ��N��O��C��EԪ�ػ�̬ԭ�Ӽ����Ų�ʽΪ��[Ar]3d104s1��
��3��������CA3�ķе�Ȼ�����BA4�ĸߣ�����Ҫԭ���ǰ������Ӽ��γ��˷��Ӽ������
��4��Ԫ��B��D�γɵ�һ�ֻ�������Ԫ��C��D�γɵ�һ�ֻ����ﻥΪ�ȵ����壬Ԫ��C��D�γɻ�����ķ���ʽ��N2O��
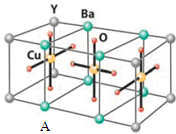
��5��Ԫ��E��һ���Ȼ��ᄃ��ľ����ṹ��ͼ��ʾ�����Ȼ���Ļ�ѧʽ��CuCl������侧���߳�Ϊacm����þ����ܶ�Ϊ$\frac{398}{{N}_{A}{•a}^{3}}$g/cm3������Ũ���ᷢ����������ԭ��Ӧ��ֻ���������HnECl3����Ӧ�Ļ�ѧ����ʽΪCuCl+2HCl��Ũ���TH2CuCl3��
���� ԭ�������������������Ԫ��A��B��C��D��E������A�����ڱ���ԭ�Ӱ뾶��СԪ�أ���AΪHԪ�أ�Bԭ�ӻ�̬ʱ���������������ڲ��������2����ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�Dԭ�ӻ�̬ʱ2pԭ�ӹ������2��δ�ɶԵĵ��ӣ�ԭ����������̼����D��������Ų�Ϊ1s22s22p4����DΪOԪ�أ�C��ԭ����������̼����֮�䣬��CΪNԪ�أ�E��ԭ������Ϊ29����EΪCuԪ�أ��ݴ˽��
��� �⣺ԭ�������������������Ԫ��A��B��C��D��E������A�����ڱ���ԭ�Ӱ뾶��СԪ�أ���AΪHԪ�أ�Bԭ�ӻ�̬ʱ���������������ڲ��������2����ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�Dԭ�ӻ�̬ʱ2pԭ�ӹ������2��δ�ɶԵĵ��ӣ�ԭ����������̼����D��������Ų�Ϊ1s22s22p4����DΪOԪ�أ�C��ԭ����������̼����֮�䣬��CΪNԪ�أ�E��ԭ������Ϊ29����EΪCuԪ�أ�
��1��C2H2���ӽṹʽΪH-C��C-H��Cԭ���γ�2���Ҽ���û�й¶Ե��ӣ���̼ԭ�ӹ�����ӻ�����Ϊsp�ӻ���1mol C2H2���ЦҼ�����ĿΪ3��6.02��1023��
�ʴ�Ϊ��sp��3��6.02��1023��
��2��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ�����Ԫ��ԭ��2p�������3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ�����ܣ�N��O��C��
E��ԭ������Ϊ29����EΪCuԪ�أ���̬ԭ�Ӽ����Ų�ʽΪ��[Ar]3d104s1��
�ʴ�Ϊ��N��O��C��[Ar]3d104s1��
��3���������Ӽ��γ��˷��Ӽ�������е����CH4��
�ʴ�Ϊ���������Ӽ��γ��˷��Ӽ������
��4��Ԫ��C��O�γɵ�һ�ֻ�������Ԫ��N��O�γɵ�һ�ֻ����ﻥΪ�ȵ����壬Ӧ��CO2��N2O��
�ʴ�Ϊ��N2O��
��5��Ԫ��E��һ���Ȼ��ᄃ��ľ����ṹ��ͼ��ʾ�������а�ɫ��Ϊ��ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����ɫ����ĿΪ4��ԭ����Ŀ֮��Ϊ1��1���ʸ��Ȼ���Ļ�ѧʽ��CuCl����������Ϊ$\frac{99.5}{{N}_{A}}$g��4������侧���߳�Ϊacm��
��þ����ܶ�Ϊ$\frac{99.5}{{N}_{A}}$g��4�£�acm��3=$\frac{398}{{N}_{A}{•a}^{3}}$g/cm3��������Ũ���ᷢ����������ԭ��Ӧ��ֻ���������HnCuCl3����Ӧ�Ļ�ѧ����ʽΪCuCl+2HCl��Ũ���TH2CuCl3��
�ʴ�Ϊ��CuCl��$\frac{398}{{N}_{A}{•a}^{3}}$��CuCl+2HCl��Ũ���TH2CuCl3��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų��������ܡ��ӻ��������ѧ�����ȵ����塢������������ȣ��ƶ�Ԫ���ǽ���ؼ�����Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

| A�� | �ٵμ�AgNO3��Һ���۲��Ƿ���AgI�������� | |
| B�� | ����CCl4�۲��²�Һ����ɫ | |
| C�� | �ٵμ�AgNO3��Һ���۲��Ƿ���AgCl�������� | |
| D�� | �ٵμ�KSCN��Һ���۲��Ƿ���Ѫ��ɫ |
| A�� | 2Q1=2Q2��Q3 | |
| B�� | ��������CO�İٷֺ�������������CO�İٷֺ����� | |
| C�� | �������з�Ӧ��ƽ�ⳣ�����������з�Ӧ��ƽ�ⳣ��С | |
| D�� | ��������CO��ת��������������CO2��ת����֮�͵���1 |
| A�� | ��aL0.1mol/L ��CH3COOH��Һ��bL0.1mol/L�� KOH��Һ��ϣ�������Һ��һ�����ڣ�c��K+��+c��H+��=c��CH3COO-��+c��OH-�� | |
| B�� | ��0.1mol/L ��NaHCO3��Һ��0.3mol/L ��Ba��OH��2��Һ�������ϣ�������Һ��һ�����ڣ�c��OH-����c��Ba+����c��Na+����c��H+�� | |
| C�� | ��1mol/L ��CH3COOH��Һ�м�������CH3COONa���壬����CH3COONaˮ���Լ��ԣ�������Һ��pH���� | |
| D�� | �����£���pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�У�ˮ�ĵ���̶���ͬ |

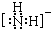 ��������ԭ�ӵ��ӻ�������sp3�ӻ�
��������ԭ�ӵ��ӻ�������sp3�ӻ� ��
�� 2001��ŵ������ѧ����������ŵ��˹��������˹���ձ���Ұ�����λ�ã����Ƿ�����ijЩ���Ի��ӿ�����ijЩ��ѧ��Ӧ�Ĵ�����Ϊ�ϳɶ��������õ���Ҫ�����↑����һ��ȫ�µ��о�����
2001��ŵ������ѧ����������ŵ��˹��������˹���ձ���Ұ�����λ�ã����Ƿ�����ijЩ���Ի��ӿ�����ijЩ��ѧ��Ӧ�Ĵ�����Ϊ�ϳɶ��������õ���Ҫ�����↑����һ��ȫ�µ��о����� ��
��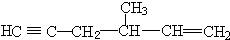 ��
�� ��
��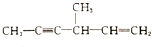 ��
��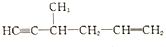
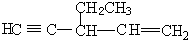 ��
�� ��
��