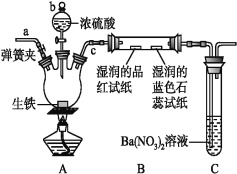
��Ŀ����
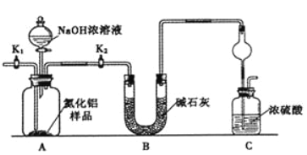
����Ŀ��������أ�K2FeO4����һ�ּ�������������������һ������Ͷ��ˮ���������������������£�
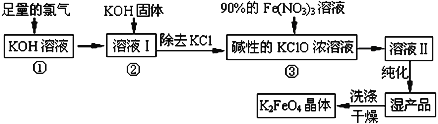
��֪����2KOH��Cl2��KCl��KClO��H2O���������¶Ƚϵͣ�
��6KOH��3Cl2 ��5KCl��KClO3��3H2O���������¶Ƚϸߣ�
�ش��������⣺
(1)����������Ӧ��______________�������¶Ƚϸ��������¶Ƚϵ�����������½��У�
(2)����ҺI�м���KOH�����Ŀ����________�����ţ���
A������ҺI�й�����Cl2������Ӧ�����ɸ����KClO
B��KOH�����ܽ�ʱ��ų��϶����������������߷�Ӧ����
C��Ϊ��һ����Ӧ�ṩ���ԵĻ���
D��ʹKClO3ת��Ϊ KClO
(3)����ҺII�з����K2FeO4�����и���ƷKNO3��KCl����Ӧ���з��������ӷ�Ӧ����ʽΪ________________________________________ ��
(4)����ж�K2FeO4�����Ѿ�ϴ�Ӹɾ�________________________________��
(5)������أ�K2FeO4����Ϊˮ��������һ���ŵ�������ˮ��Ӧ���ɽ����������ʣ���ƽ�÷�Ӧ�����ӷ���ʽ��_____________________________________________��
���𰸡��¶Ƚϵ�AC2Fe3+ + 3ClO�� + 10OH�� �� 2FeO42- + 3Cl��+ 5H2O���Թ�ȡ�������һ�ε�ϴ��Һ��������������Һ���ް�ɫ�������ѱ�ϴ��4FeO42- + 10H2O == 4Fe(OH)3�����壩+ 3O2�� + 8OH��
��������
����������Һ�г����������������¶Ƚϵ͵������·�Ӧ�����Ȼ��ء�������غ�ˮ�������������ع����ȥKCl���õ�����KClO��Ũ��Һ������90%��������Һ������Ӧ���ɸ��������Һ���õ�ʪ��Ʒ��ϴ�Ӹ���õ�K2FeO4���壬�Դ˽��
��1���ɹ������̼��ۿ�֪��������������KClO�Ʊ�������أ�����Ϣ�ڿ�֪�¶Ƚϸ�KOH��������Ӧ���ɵ�������ء�����Ϣ�ٿ�֪���ڵ�����KOH��������Ӧ���ɵ���KClO����ѡ���¶Ƚϵͣ�
��ˣ�������ȷ�������¶Ƚϵͣ�
��2���ɹ������̿�֪����ӦҺI���й�����������Ӧ����KOH�����Ŀ���������������������Ӧ�����ɸ����KClO��
A.�����������������Ӧ�����ɸ����KClO����A��ȷ��
B.����Ϣ�ڿ�֪�¶Ƚϸ�KOH��������Ӧ���ɵ�������أ�������KClO���ɡ��˷�ԭ�ϣ���B����
C.�ɹ������̼��ۿ�֪��������������KClO�Ʊ�������أ���C��ȷ��
D.����Ϣ��֪�������������������ת��ΪKClO����D����
��ѡA��C��
��ˣ�������ȷ������AC��
��3������Ŀ��Ϣ��������ԭ��Ӧ�л��ϼ۷����仯ȷ���ҳ���Ӧ�Fe3+��ClO-�������FeO42-��Cl-�����ݵ��ӵ�ʧ�غ�������غ�����ƽ����Ӧ���з��������ӷ�Ӧ����ʽΪ2Fe3+ + 3ClO�� + 10OH�� �� 2 FeO42-+ 3Cl��+ 5H2O��
��ˣ�������ȷ������2Fe3+ + 3ClO�� + 10OH�� ��2FeO42- + 3Cl��+ 5H2O��
��4����ֻҪ�������һ�ε�ϴ������Cl-������֤��K2FeO4�����Ѿ�ϴ�Ӹɾ���
�ʴ�Ϊ�����Թ�ȡ�������һ�ε�ϴ��Һ��������������Һ���ް�ɫ�������ѱ�ϴ����
��5��������أ�K2FeO4����ˮ��Ӧʱ�����������������������������ʣ���Ԫ�ر���ԭ�����ж���������ΪO2,���ݵ��ӵ�ʧ�غ�������غ�����ƽ���ɵ�4FeO42- + 10H2O = 4Fe(OH)3�����壩+ 3O2�� + 8OH����
��ˣ�������ȷ������4FeO42- + 10H2O = 4Fe(OH)3�����壩+ 3O2�� + 8OH����

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�