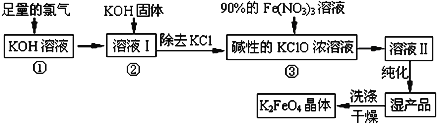
��Ŀ����
����Ŀ��ʵ������Ҫ0.1 mol��L��1NaOH��Һ450 mL��0.5 mol��L��1������Һ500 mL��������������Һ����������ش��������⣺
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������________(����������)��

(2)���в����У�����ƿ�����߱��Ĺ�����________(�����)��
A������һ�����ȷŨ�ȵı���Һ B��������Һ
C����������ƿ������µ����������Һ�� D��ȷϡ��ijһŨ�ȵ���Һ
E�����������ܽ��������
(3)���ݼ�����������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ����NaOH��Һ��ת��������ƿʱδ����ȴ����������ҺŨ��________������ڡ������ڡ���С�ڡ���0.1 mol��L��1��
(4)���ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g��cm��3��Ũ��������Ϊ________mL(����������һλС��)��
���𰸡�AC���������ձ�BCE2.0����13.6
��������
��1�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ������ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ����Բ���Ҫ��������AC������Ҫ���������ձ��Ͳ��������ʴ�Ϊ��AC�����������ձ���
��2������ƿ�����������桢������Һ���ܽ�������ʵȣ�ֻ��1���̶��߲��ܲ�������ƿ������µ����������Һ�壬�ʴ�Ϊ��BCE��
��3��m=nM=CVM=0.1mol/L��0.5L��40g/mol=2g��������ƿ�ж���ʱ��������ƿ�̶��ߣ�������Һ�����ƫС������������Һ��Ũ��ƫ�ʴ�Ϊ��2.0�����ڣ�
��4��Ũ��������ʵ���Ũ��Ϊ��c=![]() =
=![]() mol/L=18.4mol/L������ҪŨ��������ΪV��0.5mol/L��0.5L=18.4mol/L��V��V=0.0136L=13.6mL���ʴ�Ϊ��13.6��
mol/L=18.4mol/L������ҪŨ��������ΪV��0.5mol/L��0.5L=18.4mol/L��V��V=0.0136L=13.6mL���ʴ�Ϊ��13.6��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����Ŀ������β��������̼��Ҫԭ��Ϊ2NO(g)+2CO(g)![]() N2(g)+2CO2(g) +Q��Q>0����һ���������ܱ�����
N2(g)+2CO2(g) +Q��Q>0����һ���������ܱ�����
�У��ô�������ø÷�Ӧ�ڲ�ͬʱ�� NO �� CO Ũ�����±���
ʱ��/s | 0 | 1 | 2 | 3 | 4 |
c(NO)/molL-1 | 9.50��10-3 | 4.50��10-3 | 2.50��10-3 | 1.50��10-3 | 1.50��10-3 |
c(CO)/molL-1 | 9.00��10-3 | 4.00��10-3 | 2.00��10-3 | 1.00��10-3 | 1.00��10-3 |
���������գ�
��1�� д���÷�Ӧ��ƽ�ⳣ������ʽ��_________�� �¶����ߣ�������̼�����Ũ�Ƚ�______����������С�����䡱����
��2��ǰ 2s �ڵĵ�����ƽ����Ӧ�����ǣ�v(N2)=______mol/(Ls)���ﵽƽ��ʱ��CO ��ת����Ϊ��_____��
��3����ҵ�ϳ����á� ���³����������������� ������ͬʱ���� SO2 �͵������������壨NOx �����Ի��(NH4)2SO4 ��ϡ��Һ���ڴ���Һ�У�ˮ�ĵ���̶����ܵ���_____����ٽ����������ơ���û��Ӱ�족����
����(NH4)2SO4��Һ���ټ�������ϡ���ᣬ��![]() ֵ��_____����������С�����䡱����
ֵ��_____����������С�����䡱����
��4������� BaCl2 ��Һ��ͨ������ SO2 ���壬û�г������ɣ������μ�һ�����İ�ˮ��������ɰ�ɫ��������ƽ���ƶ�ԭ��������������___________________________��
��5������һ�ֿ����Ա�����Һ��ͨ������ SO2 ���壬������������ɫ�������ó����Ļ�ѧʽΪ_____�� ԭ�����Ա��ο�����_____��