��Ŀ����
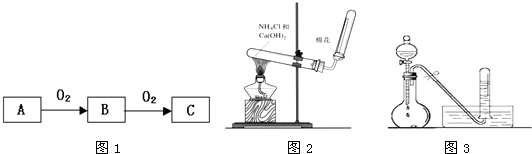
��16�֣�A��B��C����ѧ��ѧ�г��������ֶ�����Ԫ�ء���֪����AԪ��ԭ�������������Ǵ�����������2������BԪ���������������۵Ĵ�����Ϊ2����CԪ���ж��ֻ��ϼۣ��ҳ�����CԪ�صĵ�����ij��һԪǿ����Һ��Ӧ���ɵõ����ֺ�CԪ�صĻ������B��C����Ԫ��������֮����AԪ����������4����
(1) д��AԪ�������ڱ��е�λ��_______________________��
(2) д��C�ĵ��ʺ�ǿ����Һ��Ӧ�����ӷ���ʽ____________________________________��
(3) �����������ѧ��FuNvio Cacace���˻���˼��������о������B4��̬���ӡ�B4���ӽṹ������ӽṹ���� ����֪����1 mol B��B����167
kJ������������1 mol B��B�ų�942 kJ��������д����B4��̬���ӱ��B2��̬���ӵ��Ȼ�ѧ����ʽ��____________________________��
����֪����1 mol B��B����167
kJ������������1 mol B��B�ų�942 kJ��������д����B4��̬���ӱ��B2��̬���ӵ��Ȼ�ѧ����ʽ��____________________________��
(4) ��B��C����Ԫ����ɵĻ�����X��������Ϊ�ӷ��ĵ���ɫҺ�壬X����Ϊ������
���ӣ��ҷ�����B��C����ԭ���������ﵽ8�����ӵ��ȶ��ṹ��X��ˮ�������γ�һ�ֳ�����Ư�������ʡ���X�ĽṹʽΪ____________��X��ˮ��Ӧ�Ļ�ѧ����ʽ��___________________��
(5) A��B����Ԫ�ؿ��γ�һ��Ӳ�ȱȽ��ʯ����Ļ�����Y���ڻ�����Y�У�A��Bԭ�Ӽ��Ե������ϣ���ÿ��ԭ�ӵ��������ﵽ8�����ӵ��ȶ��ṹ����Y�Ļ�ѧʽΪ______________��Y������۵�Ƚ��ʯ�۵�______����ߡ��͡�)��
(6) B2H4��һ�ֿ�ȼ��Һ�壬��ˮ��Һ�������ԣ�����Ϊ��Һ�д���ƽ�⣺
H2B��BH2��H2O _________________________��
_________________________��
ÿ��2�֣���16��
(1) �ڶ����ڢ�A�� (2 ) C12��2OH��=ClO����H2O
(3) ��4(g) ====2��2(g) ��H=��882 kJ/mol
(4)  NCl3��3H2O==== NH3��3HClO
NCl3��3H2O==== NH3��3HClO
(5) C3N4 �� (6) H2N��NH+3��OH������N2H+5��OH�� ��
��������������A��B��C�ֱ���C��N��Cl��AԪ�������ڱ��е�λ���ǵڶ����ڢ�A�壻C�ĵ��ʺ�ǿ����Һ��Ӧ�����ӷ���ʽC12��2OH��=ClO����H2O��Cl- �����������4(g) ����2��2(g)����Ҫ����6 mol 1 mol B��B����1002kJ������������,2 mol B��B�ų�1884kJ���������ų�882 kJ����������4(g) ====2��2(g) ��H=��882 kJ/mol��
(4)��N��Cl�γ������η��ӣ����Ҷ��ﵽ8�����ӵ��ȶ��ṹ����ѧʽΪNCl3���ṹʽΪ ��X��ˮ��Ӧ�Ļ�ѧ����ʽ��NCl3��3H2O==== NH3��3HClO ��
��X��ˮ��Ӧ�Ļ�ѧ����ʽ��NCl3��3H2O==== NH3��3HClO ��
(5)��A��Bÿ��ԭ�ӵ��������ﵽ8�����ӵ��ȶ��ṹ��A��Bԭ�Ӽ��Ե������ϣ�
����Y�� C3N4���۵�Ƚ��ʯ�۵�ߡ�
(6) H2N��NH+3��OH������N2H+5��OH�� ��

 ����ѧ����ϵ�д�
����ѧ����ϵ�д�


 HS-+OH-
HS-+OH- Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״�������� ��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��
��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��