��Ŀ����
����Ŀ����������������Դ�����ˮ�����Ⱦ�ǻ�����������Ҫ�о����⡣
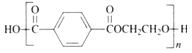
��.��ѧ�ϲ���![]() ����
����![]() ��������������Ⱦ��������Ϊ��ҵ������������Դ��
��������������Ⱦ��������Ϊ��ҵ������������Դ��
��1����ҵ�Ͽɲ���![]() �ķ����ϳɰ����ҹ�ѧ�߲���������ѧ������ͨ�������ģ�⣬�о����ڴ��������Ϻϳɰ��ķ�Ӧ���̡�ͼ1Ϊ������Ӧ�������仯ʾ��ͼ�����С�������ʾ����̬�����ڴ�������������
�ķ����ϳɰ����ҹ�ѧ�߲���������ѧ������ͨ�������ģ�⣬�о����ڴ��������Ϻϳɰ��ķ�Ӧ���̡�ͼ1Ϊ������Ӧ�������仯ʾ��ͼ�����С�������ʾ����̬�����ڴ�������������
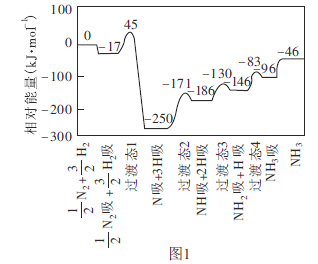
�ٸ÷�Ӧ����������Ӧ�������Ϊ________![]() ��
��
�ںϳɰ���Ӧ���Ȼ�ѧ����ʽΪ_________________��
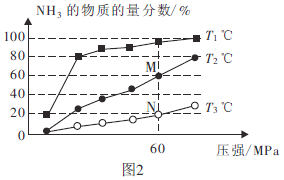
��2����ͬ�¶��£������������зֱ�Ͷ����ͬ���ķ�Ӧ����з�Ӧ����ò�ͬѹǿ��ƽ��������![]() �����ʵ���������ͼ2��ʾ��
�����ʵ���������ͼ2��ʾ��
��M���![]() ________N���
________N���![]() ���>������<����=������
���>������<����=������
��![]() ��ʱ����
��ʱ����![]()
![]() ��
��![]()
![]() ����2L���ܱ������У�ά��ѹǿΪ60MPa���䣬�ﵽN���ƽ��״̬����ѧƽ�ⳣ��K=________
����2L���ܱ������У�ά��ѹǿΪ60MPa���䣬�ﵽN���ƽ��״̬����ѧƽ�ⳣ��K=________![]() ������������ʾ����
������������ʾ����
��.��ˮ�еĵ����Ժ����л�����������μ��������ε���ʽ���ڡ�
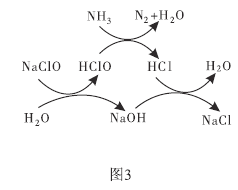
��3�����ô������Ƴ�ȥ��������![]() ʾ����ԭ����ͼ3��ʾ��д���ܷ�Ӧ�Ļ�ѧ����ʽ��______________��
ʾ����ԭ����ͼ3��ʾ��д���ܷ�Ӧ�Ļ�ѧ����ʽ��______________��
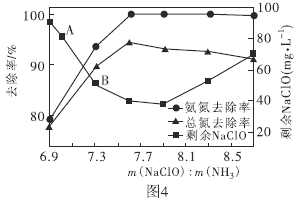
��ȡһ�����ĺ�������ˮ���ı����������Ƶ���������Ӧ��ͬʱ�����Һ�а���ȥ���ʡ��ܵ�����Һ�����п����Եĺ����������е�Ԫ�ص�������ȥ�����Լ�ʣ��������Ƶĺ�����![]() �ı仯�����ͼ4��ʾ����
�ı仯�����ͼ4��ʾ����![]() ʱ��ˮ�����ܵ�ȥ���ʷ����½������ܵ�ԭ����________��
ʱ��ˮ�����ܵ�ȥ���ʷ����½������ܵ�ԭ����________��
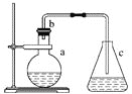
��4����������ˮ���е������κ����������ӡ���ҵ��ȥ����ˮ�������εķ����Dz��õ�ⷨ��![]() ת��Ϊ
ת��Ϊ![]() ������װ����ͼ5��ʾ��
������װ����ͼ5��ʾ��
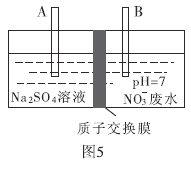
��B�缫�ĵ缫��Ӧʽ��________.
�ڳ����£���ȥ10L��ˮ�е�![]()
![]() �����շ�ˮ��
�����շ�ˮ��![]() ________�����ǰ���ˮ������仯���Բ��ƣ���
________�����ǰ���ˮ������仯���Բ��ƣ���
���𰸡�79 ![]()
![]() <
< ![]()
![]() ���
���![]() ��������
��������![]() ��
��![]()
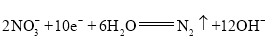 10
10
��������
��1������ͼ��֪������Ӧ�����Ļ��Ϊ![]() ���ʴ�Ϊ��79��
���ʴ�Ϊ��79��
�ڷ�Ӧ��=������������-��Ӧ��������![]() �ϳɰ���Ӧ���Ȼ�ѧ����ʽΪ
�ϳɰ���Ӧ���Ȼ�ѧ����ʽΪ![]()
![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]()
![]() ��
��
��2����M��N�����ѹǿ��ͬ��ƽ�������а��������ʵ�������M>N����Ϊ�ϳɰ��Ƿ��ȷ�Ӧ������ƽ�������ƶ�������![]() ��M���
��M���![]() ���
���![]() ���ʴ�Ϊ��<��
���ʴ�Ϊ��<��
���������ĵĵ��������ʵ���Ϊxmol��
![]()
��ʼ����mo1����1 3 0
ת������mo1����x 3x 2x
ƽ������mol����1-x 3-3x 2x
ƽ��ʱ�����������ʵ�������Ϊ20%����![]() ����
����![]() ��
��
��ƽ��ʱ��![]() ��
��![]() ��
��![]()
�����ʵ���Ϊ![]() ����ѹʱ�������֮�ȵ������ʵ���֮�ȣ�
����ѹʱ�������֮�ȵ������ʵ���֮�ȣ�
����ƽ��ʱ�������Ϊ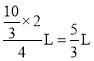 ��
��![]() ��
��![]() ��
��
��ƽ�ⳣ��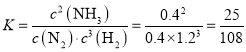 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��3���ٸ���ͼʾ��֪��Ӧ��Ϊ![]() ��NaClO�����ղ���Ϊ
��NaClO�����ղ���Ϊ![]() ��NaC1��
��NaC1��![]() ��������ƽ���ɵó���ѧ����ʽ
��������ƽ���ɵó���ѧ����ʽ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
�ڵ�![]() ʱ��������NaClOͶ�����Ὣ��������Ϊ
ʱ��������NaClOͶ�����Ὣ��������Ϊ![]() ��
��![]() �����ʣ�δ�ܴ���Һ�г�ȥ��ʹ���ܵ�ȥ������
�����ʣ�δ�ܴ���Һ�г�ȥ��ʹ���ܵ�ȥ������![]() ����������͡��ʴ�Ϊ���в���
����������͡��ʴ�Ϊ���в���![]() ��������
��������![]() ��
��![]() ��
��
��4����B�缫������ԭ��Ӧ��![]() ת��Ϊ
ת��Ϊ![]() ��
��
��62mg![]() �����ʵ���Ϊ0.001mol������B�缫�ĵ缫��Ӧʽ
�����ʵ���Ϊ0.001mol������B�缫�ĵ缫��Ӧʽ![]() ��֪ת��
��֪ת��![]()
![]() ������
������![]()
![]() ��A���ĵ缫��ӦʽΪ
��A���ĵ缫��ӦʽΪ![]() ��ת��
��ת��![]()
![]() ������0.005mol H+��H+�ɵ��ص���������ұߣ�����
������0.005mol H+��H+�ɵ��ص���������ұߣ�����![]() �����
�����![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��10��
��10��

����Ŀ��������������������![]() ������Ҫ����ԭ�ϣ�Ҳ��һ����ɫ��̬ɱ���������Ʒ�Ϊ
������Ҫ����ԭ�ϣ�Ҳ��һ����ɫ��̬ɱ���������Ʒ�Ϊ![]() ��
��
��1��ijͬѧд����̼ԭ�ӵ�4�ֲ�ͬ״̬�ĵ����Ų�ͼ��
a.![]() b.
b.![]()
c.![]() d.
d.![]()
����������͵���________�����ţ���������״̬C��״̬B���õ��Ĺ���Ϊ________���ף��ԭ�ӷ��䡱��ԭ�����ա�����
��2���������������Cԭ�ӵ��ӻ���ʽ��________��
��3��������Ҵ������![]() ��ԭ��____________________��
��ԭ��____________________��
��4���۵㣺����________���ᣨ�>������<����=����������_________________��
��5����ֽ�У���![]() ��ֽ���еĹ��������Լ����ɽ������ӷ�Ӧ�����Ư��Ч�ʡ����⻯�ơ����⻯������Ϊ���л���ѧ�ϵġ����ܻ�ԭ������
��ֽ���еĹ��������Լ����ɽ������ӷ�Ӧ�����Ư��Ч�ʡ����⻯�ơ����⻯������Ϊ���л���ѧ�ϵġ����ܻ�ԭ������
���������⻯����۵����±���ʾ��
���⻯�� |
|
|
�۵�/�� | 400 | -64.5 |
���ͱ������������۵�����ԭ��________��
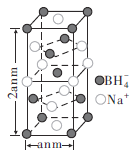
�����⻯�ƾ����ṹ��ͼ��ʾ���þ�����![]() ����λ��Ϊ________����֪�����⻯�ƾ�����ܶ�Ϊ
����λ��Ϊ________����֪�����⻯�ƾ�����ܶ�Ϊ![]() ��
��![]() ��������٤��������ֵ����a=________nm���ú�
��������٤��������ֵ����a=________nm���ú�![]() ��
��![]() �����ʽ�ӱ�ʾ����
�����ʽ�ӱ�ʾ����