��Ŀ����
���������μ����ֽ��Ҳ���ϸ��ӣ�ijѧϰС����Fe��NO3��2Ϊ�о�����ͨ��ʵ��̽�����ȷֽ�IJ���������ϣ��������������ȷֽ�Ĺ��ɣ��������4�ֲ��룺
�ף�Fe2O3��NO2�ң�Fe2O3��NO2��O2����Fe3O4��NO2��O2����FeO��NO2��N2
��1����ʵ��ǰ��С���Ա�������϶����붡��������������__________________��
����������룬�����ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���
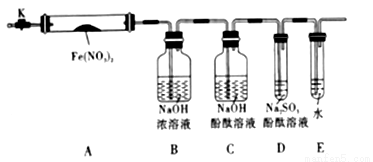
��2����ʵ�����
���������Ӻ��˹����Լ�֮ǰ��Ӧ______________
�ڳ�ȡFe��NO3��2����3.6g����A�У�����ǰ�ȴ�K��___________�����ٹر�K���þƾ��Ƽ���
�۹۲쵽A���к���ɫ������֣�C��D��δ�����Ա仯��
�ܴ���Ʒ��ȫ�ֽ⣬Aװ����ȴ�����¡����������ʣ����������Ϊ1.6g
��ȡ����ʣ��������Թ��У���������ˮ��δ����������
��3����ʵ������������:
�ٸ���ʵ�������ʣ����������������,�ɳ���ȷ�Ϸֽ��������____________��
�ڸ���D������������һλͬѧ��Ϊ����ȷ�Ϸֽ��������O2����Ϊ����O2��D����Һ��ɫ����ȥ����һ��ͬѧ�϶��ֽ��������O2���ڣ�δ��ൽ��ԭ����_________________��
��Ϊ����֤�Ƿ���O2��ijͬѧ����Fe��NO3��2����3.6g����ּ��ȣ��ռ����壬�������������ȫ���ռ���������������Թܵ�����ˮ���У��۲쵽�������Ǻ���ɫ��ʧ��Һ�������������Թ��е���Һ����2/3��ͨ��һ������O2������ȫ�������գ���д��Fe��NO3��2���ȷֽ�Ļ�ѧ����ʽ______________,ͨ��O2�����ʵ���Ϊ_____________��
��С�����ۺ��ɵĹ�ʶ������ʵ������Բ����ƣ���Ľ�װ�ý�һ���о���дһ�㲻��֮����__________________________��
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�| A�� | ����ͬ�����µ��ܽ�ȣ�NaHCO3��Na2CO3 | |
| B�� | ���ȶ��ԣ�HF��HCl��HBr��HI | |
| C�� | �����ԣ�F2��Cl2��Br2��I2 | |
| D�� | ̼������Һ��ϡ���ụ��ʱ�����ֲ���������ͬ |
| A�� | Ʒ����Һ��ɫ | B�� | AgNO3��Һ�������� | ||
| C�� | BaCl2��Һ�������� | D�� | ʯ����Һ��� |




 3Mg+Al2O3
3Mg+Al2O3 K+��Cl-
K+��Cl- ��Һ�У�Fe2+��NH4+��SO32-��Cl-
��Һ�У�Fe2+��NH4+��SO32-��Cl-