��Ŀ����
5���±���ʵ����Ԫ�����ڱ��IJ��ֱ߽磬�����ϱ߽粢δ��ʵ������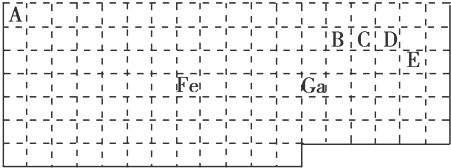
������Ϣ�ش��������⣺
��1����̬Gaԭ�ӵ����������Ų�ʽΪ4s24p1
��2����Ԫ��λ��Ԫ�����ڱ���d����Fe��CO���γ������Fe��CO��5����Fe��CO��5�����Ļ��ϼ�Ϊ0��
��3����֪��ԭ����Ŀ�ͼ۵�����������ͬ������Ϊ�ȵ����壬�ȵ�����������ƵĽṹ��������CO��Ϊ�ȵ�����ķ��Ӻ����ӷֱ�ΪN2��CN-���ѧʽ����
��4����CH4��CO��CH3OH�У�̼ԭ�Ӳ�ȡsp3�ӻ��ķ�����CH4��CH3OH��
��5������VSEPRģ��Ԥ��ED4-�Ŀռ乹��Ϊ�������壮B��C��D��Eԭ��������γɵķ����У�����ԭ�Ӷ����������8�����ȶ��ṹ�ķ���ΪCO2 CCl4��д2�֣���
���� ��1��Gaλ�ڵ������ڵ�IIIA�壬�������3�����ӣ�
��2��Ԫ�����ڱ������Ļ����Ǹ����������ĵ������������ģ����ݻ�����Ļ��ϼ۴�����Ϊ0������
��3���۵�������ͬ��ԭ������ͬ�������Ƶȵ����壻
��4���ȸ��ݼ۲���ӶԻ��������жϼ۲���Ӷԣ�Ȼ����ȷ����ȡ���ӻ���ʽ��
��5���۲���Ӷ���=�Ҽ����Ӷ���+����ԭ���ϵŵ��Ӷ��������ݼ۲���Ӷ����ж������ͣ�����Ԫ�����ڱ�֪��B��C��D��E�ֱ���C��N��O��Cl������Ԫ�أ������ϼ۵ľ���ֵ+ԭ������������=8��������ÿ��ԭ������㶼�ﵽ8�����ȶ��ṹ���ݴ˷�����
��� �⣺��1��Gaλ�ڵ������ڵ�IIIA�壬�������3�����ӣ������������Ų�ʽΪ4s24p1��
�ʴ�Ϊ��4s24p1��
��2��Ԫ�����ڱ������Ļ����Ǹ����������ĵ������������ģ�Feԭ����26��Ԫ�أ�Feԭ�ӵĻ�̬��������Ų�ʽΪ��1s22s22p63s23p63d64s2��
��Щ��������ʱ��ѭ�������ԭ�������������͵ĺ��������ߵģ�����Щ�ܼ���������С˳��Ϊ��1s��2s��2p��3s��3p��4s��3d��
�����������ԭ������4s���Ӻ���3d���ӣ����� ����������3d���ӣ���������������d�������ݻ����ﻯ�ϼ۵Ĵ�����Ϊ0��Fe��CO���γ������Fe��CO��5���ϼ۵Ĵ�����Ϊ0��CO�Ļ��ϼ۵Ĵ�����Ϊ0���ʴ�Ϊ��d��0��
��3�����ݵȵ�����Ķ��壬CO�ĵȵ����������˫ԭ�ӷ��ӻ����ӣ��ҵ���������ȣ�����ԭ�ӣ�ԭ������=ԭ�Ӻ���������������ԣ�����Ƿ��ӣ�ֻҪԭ������֮����ȼ��ɣ�CO��C��6��Ԫ�أ�O��8��Ԫ�أ�ԭ������֮��Ϊ14��N��7��Ԫ�أ��ҿ��γ�˫ԭ�ӷ��ӣ����Է�����N2��
�ʴ�Ϊ��N2��CN-��
��4����CH4�м۲���Ӷ���=�Ҽ����Ӷ�����4��+����ԭ���ϵŵ��Ӷ�����0��������̼ԭ�Ӳ�ȡsp3�ӻ�����CO�����м۲���Ӷ���=�Ҽ����Ӷ�����1��+����ԭ���ϵŵ��Ӷ�����1��������̼ԭ�Ӳ�ȡsp�ӻ���CH3OH�У��۲���Ӷ���=�Ҽ����Ӷ�����4��+����ԭ���ϵŵ��Ӷ�����0��������̼ԭ�Ӳ�ȡsp3�ӻ���
�ʴ�Ϊ��CH4��CH3OH��
��5������Ԫ�����ڱ�֪��E��ClԪ�أ�D��OԪ�أ�ED4-��ClO4-���ӣ��۲���Ӷ���=�Ҽ����Ӷ���+����ԭ���ϵŵ��Ӷ������۲���Ӷ���=4+$\frac{1}{2}$��7+1-4��2��=4������VSEPR����Ԥ��ED4-���ӵĿռ乹��Ϊ���������ͣ�
C��N��O��Cl������Ԫ�أ������γɵĻ�������ÿ��ԭ������㶼�ﵽ8�����ȶ��ṹ�Ļ������У�CO2 CCl4���ʴ�Ϊ���������壻CO2 CCl4��
���� ���⿼���̬ԭ�ӻ����ӵĺ�������Ų������������Ų���������ԭ�Ӳ�ȡ���ӻ���ʽ������Ԫ�����ڱ���ѧϰʱҪ��ס�������ڡ���Ļ��֣�Ҫ���ǰ36��Ԫ�أ������Ļ��֡��߽硢�������ݵȶ�Ҫ���գ���ѧϰ��Ҫ������ո���VSEPR����Ԥ����ӻ����ӵĿռ乹����ѧϰ���ص㣬Ҳ�ǽ�����߿����ȵ㣮

��1 ��2 ��3 ��4 ��5��
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �ۢ� |
| A�� | CaO | B�� | AlCl3 | C�� | NaOH | D�� | CaCl2 |
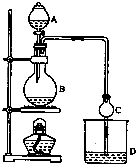 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2•6C2H5OH
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
��1��Ũ��������������ԡ���������ˮ��������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ��CH3CO18OH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOC2H5+H218O��
��2�����θ����C�������Ƿ�ֹ����������������Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ����Ӧ������D�е���������Һ�ֲ㣬�ϲ���ɫ����Һ�壬�²���Һ��ɫ��dz��
��3�����÷�Һ���������ƣ����� D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ȼ�������ˮ�Ȼ��ƣ�������Ҵ����ټ��루������ѡ����ѡ��C��Ȼ����������ռ�77�����ҵ���֣��Եýϴ���������������
A������������ B����ʯ�� C����ˮ������ D����ʯ�ң�
| A�� | m��n | B�� | m-2��10-n | C�� | m+2��n+10 | D�� | m+8��n+10 |
| A�� | H2CO3+H2O?H3O++HCO3- | B�� | HCO3-+H2O?OH-+H2CO3 | ||
| C�� | HCO3-+OH-?H2O+CO32- | D�� | HCO3-+H2O?H3O++CO32- |
 ���Ϲ�����仯�����2009��12��7-18���ڸ籾�����ٿ����й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%��45%��
���Ϲ�����仯�����2009��12��7-18���ڸ籾�����ٿ����й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%��45%��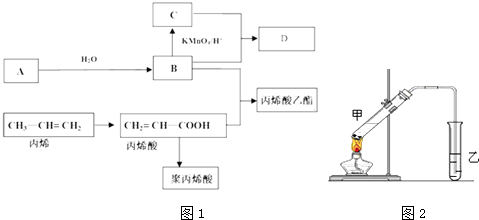
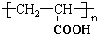 ��
��