��Ŀ����
6��������һ����Ҫ�Ļ�����Ʒ����ҵ�Ͽ�������ͼ��ʾ��������������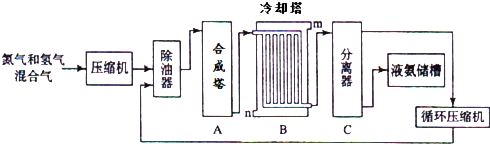
��1��ԭ����֮һ�����Ĺ�ҵ��ȡ�����Ƿ���Һ̬������д�������Ĺ�ҵ��;���δ�һ�㣩�����������������Ƶ��ʣ�
��2��д���ϳ����з����ķ�Ӧ�Ļ�ѧ��Ӧ����ʽN2+3H2$?_{���¸�ѹ}^{����}$2NH3��
����ȴ���жԻ�����������ȴ����ˮ�����n����m��n����
��3���豸C�����ý����ɵ�Һ̬������δ��Ӧ��ԭ�������룮
��������еĹ��̶�������ҵ�ϳɰ������就�����ϱ�Һ�����룬��С������Ũ�ȣ���ʹƽ�ⲻ�������ƶ������ԭ�ϵ������ʣ����Խ��ƽ���ƶ�ԭ���ش�
��4����ԭ�����Ʊ������л��� CO�Դ����ж������ã�����ȥԭ�����е� CO����ͨ�����·�Ӧ��ʵ�֣�CO��g��+H2O��g��?CO2��g��+H2��g������֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������80%������ʼ����c��H2O����c��CO��������5.9����ȷ��С�����һλ����
���� ��1�������еĽ�����ĵ��ǵ��������Թ�ҵ���÷���Һ̬�����ķ����õ����������ݰ���������ȷ����;��
��2����ҵ�ϳɰ��кϳ����еķ�ӦΪ�����������ڸ��¸�ѹ���������ɰ��������������ķ�ʽ��ȴЧ���ã�
��3���豸C�Ƿ������������ɰ�����ʱ���������������ƽ�������ƶ���
��4������K=$\frac{c��{H}_{2}��c��C{O}_{2}��}{c��CO��c��{H}_{2}O��}$�����CO��ת���ʽ��м��㣮
��� �⣺��1�������еĽ�����ĵ��ǵ��������Թ�ҵ���÷���Һ̬�����ķ����õ��������������������������������Ƶ��ʣ�
�ʴ�Ϊ������Һ̬�����������������������Ƶ��ʣ�
��2����ҵ�ϳɰ��кϳ����еķ�ӦΪ�����������ڸ��¸�ѹ���������ɰ�������Ӧ����ʽΪN2+3H2$?_{���¸�ѹ}^{����}$2NH3�����������ķ�ʽ��ȴЧ���ã�������ˮ��n�����룬
�ʴ�Ϊ��N2+3H2$?_{���¸�ѹ}^{����}$2NH3��n��
��3���豸C�Ƿ������������ɵ�Һ̬������δ��Ӧ��ԭ�������룬�������ϱ�Һ�����룬��С������Ũ�ȣ���ʹƽ�ⲻ�������ƶ������ԭ�ϵ������ʣ�
�ʴ�Ϊ�������ɵ�Һ̬������δ��Ӧ��ԭ�������룻�������ϱ�Һ�����룬��С������Ũ�ȣ���ʹƽ�ⲻ�������ƶ������ԭ�ϵ������ʣ�
��4���迪ʼCOΪamol/L��H2OΪbmol/L��
CO��g��+H2O��g��?CO2��g��+H2��g����
a b
0.2a b-0.8a 0.8a 0.8a
����K=$\frac{c��{H}_{2}��c��C{O}_{2}��}{c��CO��c��{H}_{2}O��}$=$\frac{0.8a��0.8a}{0.2a����b-0.8a��}$=0.627������b��a=5.9��������ʼ����c��H2O����c��CO��������5.9��
�ʴ�Ϊ��5.9��
���� ���⿼���˺ϳɰ���ԭ������ѧƽ��ļ����֪ʶ���ۺ��Խ�ǿ���е��Ѷȣ�ע�⻯ѧƽ��ԭ���ڹ�ҵ�������е�Ӧ�ã�

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�| A�� | ����������һ���ǽ�������������������ﲻһ�����Ƿǽ��������� | |
| B�� | ����������Ӿ��������Ϊ���������������Ӷ��Ե��� | |
| C�� | �������ǵ���ʵı���������ˮ��Һ�ܷ� | |
| D�� | ���ӷ�Ӧ�Ƿ��ܹ�����Ҫ������֮���ܷ������ֽⷴӦ |
| A�� | ���ֿڷ�Һ���ḻ�ĵ����ס�п����Ԫ�� | |
| B�� | ���������в����κλ�ѧ���� | |
| C�� | ��������ˮ���Դ��������в����κ����� | |
| D�� | û��ˮ��û������ |
| A�� | ʳ�ﴢ���ڱ����� | |
| B�� | ��MnO2��H2O2�ֽⷴӦ�Ĵ��� | |
| C�� | �ÿ�״̼��ƴ����ĩ״̼�����ϡ���ᷴӦ | |
| D�� | ��1 mol/L H2SO4��Һ����3 mol/L H2SO4��Һ��п����Ӧ |
| A�� | ������Al3+����Һ�У�Na+��AlO2-��SO42-��I- | |
| B�� | ����ʹpH��ֽ������ɫ����Һ�У�Na+��S2-��NO3-��CO32- | |
| C�� | ��ˮ�������c��OH-��=10-13mol•L-1����Һ�У�Na+��AlO2-��S2-��CO32- | |
| D�� | c��H+��=10-14mol•L-1����Һ�У�Mg2+��NO3-��Fe2+��ClO- |
| A�� | ����ϩ������ | B�� | �ɼ��������Ȼ�̼ | ||
| C�� | ������������ȼ�� | D�� | ���Ҵ�����ϩ |
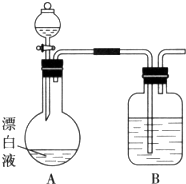 ʵ��С��ͬѧ����һ����ij��ֽ���۳���Ư��Һ��NaCl��NaClO�Ļ��Һ������ʢ�ű���KAl��SO4��2��Һ�ij��У�����ж��¼�����С��ͬѧΪ̽���ж�ԭ�����������ʵ�飮
ʵ��С��ͬѧ����һ����ij��ֽ���۳���Ư��Һ��NaCl��NaClO�Ļ��Һ������ʢ�ű���KAl��SO4��2��Һ�ij��У�����ж��¼�����С��ͬѧΪ̽���ж�ԭ�����������ʵ�飮