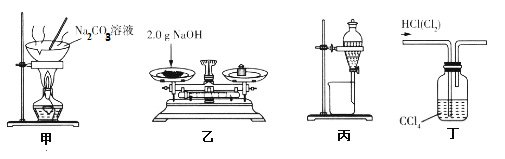
��Ŀ����
����Ŀ����������(CHBr2��CHBr2)��һ����ɫ��Һ�壬�ܶ�2.967g��mL-1��������ˮ���е�244�棬�������������ϵ���Ч�����ȡ��õ�ʯ(��Ҫ�ɷ�CaC2������CaS��Ca3P2��Ca3AS2��)��Br2��Ϊԭ���Ʊ��������������װ��(�г�װ����ʡ��)��ͼ1��ʾ��

��1��װ��A��CaC2����ˮ���ҷ�����Ӧ��CaC2+2H2O��Ca(OH)2+C2H2����Ϊ�˵õ�ƽ����C2H2�����������ñ���ʳ��ˮ����ˮ�⣬���ɲ�ȡ�IJ���������__��
��2��װ��B�ɳ�ȥH2S��PH3��AsH3�����г�ȥPH3�Ļ�ѧ����ʽΪ__(PH3��ԭ�Խ�ǿ����������������)��
��3��װ��C����Һ��Һ���ϼ���һ��ˮ��Ŀ����__��װ��C�з�Ӧ����ɵ�������__����װ��C��Ӧ�����ϵ�õ���������Ʒ����Ҫ���еIJ�����__��
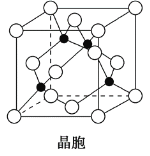
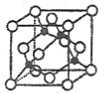
��4��һ���Ʊ�Ca10(PO4)6(OH)2��ԭ��Ϊ10Ca(OH)2+6H3PO4=Ca10(PO4)6(OH)2��+18H2O���������װ��A�õ���ʯ�����Ϊԭ���Ʊ�Ca10(PO4)6(OH)2��ʵ�鷽�������ձ��м���0.25L��0.5mol��L-1Ca(OH)2��ʯ���飬_����100������к��1h��
��֪��
��Ca10(PO4)6(OH)2��Ca/P������ֵΪ1.67��Ӱ���ƷCa/P�ȵ���Ҫ�����з�Ӧ��Ͷ�ϱȼ���ӦҺpH��
����95�棬pH��Ca/P�ȵ�Ӱ����ͼ2��ʾ��
��ʵ������ʹ�õ��Լ�����0.5mol��L-1Ca(OH)2��ʯ���顢0.3mol��L-1���ἰ����ˮ��
���𰸡���μ���(����ʳ��)ˮ 4CuSO4+PH3+4H2O=4Cu��+H3PO4+4H2SO4 ˮ�⣬����Һ��Ļӷ� ��������Һ�����Ϊ��ɫ���Ҽ�������������Ȳ�� ��Һ���л������������ռ�244����� �ڷ�Һ©���м���0.25L0.3mol��L-1���ᣬ��ʯ������ȵ�95�棬�ڲ��Ͻ����£��ȿ��ٵμ����ᣬȻ�������μӣ���ʱ�μ�����ˮ�Բ�����������ˮ�֣�ֱ������ȫ�����꣬���ڲ�������ҺpH8��9���ٳ�ֽ���һ��ʱ�䡢���ã����ˡ�ˮϴ
��������
A�е�ʯ��ˮ��Ӧ������Ȳ���������ƣ���Ϊ��ʯ�к���CaC2������CaS��Ca3P2��Ca3AS2�ȣ����Եõ�����Ȳ�к���H2S��PH3��AsH3��ԭ�����壬ͨ������ͭ��Һ����CuS��Cu��H2SO4��H3PO4��H3AsO4���Ӷ���ȥ��H2S��PH3��AsH3����ͨ��Һ�壬Һ�����Ȳ��Ӧ����1��1��2��2�������飬���������Һ��������ԭ�����塣
(1)Ϊ�˵õ�ƽ����C2H2�����������ñ���ʳ��ˮ����ˮ�⣬������ͨ���ı���뱥��ʳ��ˮ���ʿ��ƣ����Ըı�Ĵ�ʩΪ��μ���(����ʳ��)ˮ��
(2)������Ϣ֪�����������ͭ��Ӧ����Cu����������ᣬ��Ӧ����ʽΪ4CuSO4+PH3+4H2O=4Cu��+H3PO4+4H2SO4��
(3)���ж����ӷ���Ϊ��ֹ��ӷ�����Һ��ķ������岻������ˮ�����Բ���ˮ��ķ����������Ȳ��Ӧ����������ˮ��1��1��2��2���������ʹ����ɫ���������ɵ�1��1��2��2���������ˮ�ֲ㣬����������Һ�����Ϊ��ɫ���Ҽ�������������Ȳ��ʱ˵��C�з�Ӧ�Ѿ���ɣ��������ܵ�Һ����÷�Һ�������룬���ܵ�Һ������������룬����ˮ����л�����÷�Һ�������룬�л�����Һ����������������룬�ռ�244����֣�
(4)������Ϣ֪���÷�Ӧ��Ҫ95���������ʯ�����Ʊ�Ca10(PO4)6(OH)2��Һ����������ڷ�Һ©�����������٣�Ϊ�ӿ췴Ӧ������Ҫ���Ͻ��裬�ȿ��ٵμ����ᣬȻ�������μӣ�Ϊ��ֹ��Һ��ˮ�ּ��٣���Ҫ��ʱ�μ�����ˮ������������ˮ�֣�ֱ������ȫ�����꣬�ҿ�����Һ��pH��8��9���ٳ�ֽ���һ��ʱ�䡢���ã����ˡ�ˮϴ����100�������к��1 h���Ӷ��õ��ϸ����Ca10(PO4)6(OH)2.

����Ŀ�������ؾ������õĿ��������ԡ������ǰ����ص�һ�ֺϳ�·�ߣ�
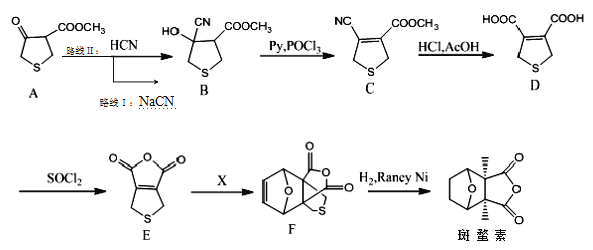
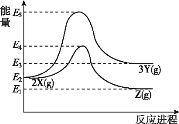
��֪����.�����������䣬ͨ����Ӧ��Ũ��Խ������Խ�죬�ಽ��Ӧʱ������������Ӧ������
��.�Ҷ�˹�����·�Ӧ��![]()
��1���л�������A�ķ���ʽΪ__��
��2��Aת��ΪBʱ��·�ߢ��·�ߢ����ˮ��Һ�н��С�ʵ�鷢�֣�·�ߢ��·�ߢ�����ʱ��̣��Խ���ԭ��__��
��3��д��B��C�Ļ�ѧ����ʽ__��
��4��D���Ҷ����������۷�Ӧ�Ļ�ѧ����ʽΪ__��
��5����X�Ľṹ��ʽ��__��
���±��о���__�Ժϳ�F��Ӱ�죬�ϳ�F�Ľ�������Ϊ__�����ţ���
��� | ����Һ��X | ��Ӧ�¶�/�� | ��Ӧʱ��/h | E����/% | F����/% | |
1 | [BPy]BF4 | 45 | 20 | 90 | 9 | |
2 | [HMIM]PF6 | 35 | 20 | 5 | 93 | |
3 | [BMIM]BF4 | 25 | 30 | 0 | 90 | |
4 | [HMIM]BF4 | 35 | 20 | 38 | 57 | |
5 | [BMIM]BF4 | 25 | 20 | 0 | 86 |
��6����д����![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�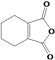 �ĺϳ�·������ͼ__�����Լ���ѡ���ϳ�·������ͼʾ��������ɣ���
�ĺϳ�·������ͼ__�����Լ���ѡ���ϳ�·������ͼʾ��������ɣ���