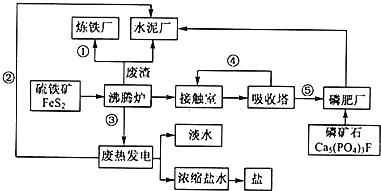
��Ŀ����
����Ŀ��ij��γ�С������50 mL NaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ�������CO2��������NaHCO3�����������ʵ�鲽�裺
a.ȡ25 mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
b.С�������Һ1��2 min�������ܽ�����Һ�е�CO2���壻
c.�ڵõ�����Һ�м�����һ��(25 mL)NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
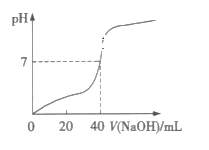
(1)�˷������Ƶýϴ�����Na2CO3��д��c��������ӷ���ʽ_________���˷�����һ����ʵ��װ����ͼ��ʾ��
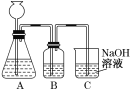
(2)���뷴Ӧ��ǰ����μ�������װ�õ������ԣ�___________��
(3)���ô���ʯ��������CO2����װ��B��ʢ�ŵ��Լ���___________�������ǣ�_________��
(4)��ʵ����ͨ���Ʒ��У�װ��A������Ϊ����_________(�����)����ķ���װ�á�
��HCl�� ��H2�� ��Cl2��
(5)��֪����NaOH��Һ�����ʵ���������Ϊ40%�������¸���Һ�ܶ�Ϊ1.44 g/mL�����跴Ӧǰ����Һ��������䣬������ʵ���������ô��ַ����Ʊ�����Na2CO3��Һ�����ʵ���Ũ��Ϊ_____mol/L
���𰸡�HCO3��OH=CO32��H2O ��ֹˮ�м�סB���ձ�֮����齺�ܣ�Ȼ���©����ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ��ʱ�䣬�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©�� ����̼��������Һ ����HCl���� �� 7.2
��������
(1)c���跢���ķ�ӦΪ̼�������ܺ��������Ʒ�Ӧ����̼���ƺ�ˮ��
(2)����Һ�������γ�Һ��߶Ȳ�������������������������װ�õ������ԣ�
(3)�����ӷ�����ȡ�Ķ�����̼����HCl��B��ʢ�ŵ��Լ���������HCl���������������̼��Ӧ�����ɶ�����̼��ã�
(4)����ȡװ���ʺϲ�������ȡ���壻
(5)����50mLNaOH��Һ�к��е��������Ƶ����ʵ�����������Ԫ���غ��֪����Һ��n(Na2CO3)��0.5n(NaOH)���ٸ���c��nV���㡣
(1)̼�������ܺ��������Ʒ�Ӧ����̼���ƺ�ˮ����Ӧ���ӷ���ʽΪHCO3��OH=CO32��H2O���ʴ�Ϊ��HCO3��OH=CO32��H2O��
(2)��ֹˮ�м�סB���ձ�֮����齺�ܣ�Ȼ���©����ע��һ������ˮ����װ�����ܷ����壬ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ�ᣬ�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©����
�ʴ�Ϊ����ֹˮ�м�סB���ձ�֮����齺�ܣ�Ȼ���©����ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ��ʱ�䣬�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©����
(3)�����ӷ�����ȡ�Ķ�����̼����HCl��B��ʢ�ű���̼��������Һ������HCl���壬ͬʱ���ɶ�����̼��
�ʴ�Ϊ������̼��������Һ������HCl���壻
(4)����ȡװ���ʺϲ�������ȡ���壬HCl��Cl2���Ʊ�����Ҫ���ȣ�����ʹ�ø�װ���Ʊ�����ȡH2����Ҫ���ȣ�����ѡ�ø�װ�ã�
�ʴ�Ϊ���ڣ�
(5)m(NaOH)��50mL��1.44g/mL��40%��28.8g������n(NaOH)��![]() ��0.72mol��������Ԫ���غ��֪����Һ��n (Na2CO3)��0.5 n(NaOH)��0.36mol����c(Na2CO3)��
��0.72mol��������Ԫ���غ��֪����Һ��n (Na2CO3)��0.5 n(NaOH)��0.36mol����c(Na2CO3)��![]() ��7.2mol/L��
��7.2mol/L��
�ʴ�Ϊ��7.2��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�����Ŀ����ʽ����(NiOOH)���÷�������(��Ҫ��Ni��Al������Cr��FeS��)���Ʊ����乤���������£�

�ش��������⣺
(1)�����ݳ�����ʱ��������Ӧ�����ӷ�Ӧ����ʽΪ_____________________��
(2)������1���õ��IJ�������________________________________________��
(3)��֪�������½������ӿ�ʼ��������ȫ������pH���±�:
��ʼ������pH | ��ȫ������pH | |
Ni2+ | 6.2 | 8.6 |
Fe2+ | 7.6 | 9.1 |
Fe3+ | 2.3 | 3.3 |
Cr3+ | 4.5 | 5.6 |
����pH 1��ʱ����ҺpH��ΧΪ_______________��
(4)�ڿ����м���Ni(OH)2�ɵ�NiOOH����д���˷�Ӧ�Ļ�ѧ����ʽ________________��
(5)��������Һ��CrO![]() ����ת����Cr2O
����ת����Cr2O![]() �������ӷ���ʽ��ʾ��ת����Ӧ__________����֪BaCrO4��Ksp=1.2��10-10��Ҫʹ��Һ��CrO
�������ӷ���ʽ��ʾ��ת����Ӧ__________����֪BaCrO4��Ksp=1.2��10-10��Ҫʹ��Һ��CrO![]() ������ȫ(c(CrO
������ȫ(c(CrO![]() )�Q1��10-5mol��L1),��Һ�б�����Ũ������Ϊ________mol��L1��
)�Q1��10-5mol��L1),��Һ�б�����Ũ������Ϊ________mol��L1��
����Ŀ����.�״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ����Һ��ȼ�ϣ���ҵ�Ͽ�����CO��CO2������ȼ�ϼ״�����֪�״��Ʊ����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�������ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶��� | |
500 | 800 | ||
��2H2(g)+CO(g) | K1 | 2.5 | 0.15 |
��H2(g)+CO2(g) | K2 | 1.0 | 2.50 |
��3H2(g)+CO2(g) | K3 | ||
��1���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3��__________����K1��K2��ʾ��
��2����Ӧ�۵���S__________0������������������������Ӧ�۵���H__________0��������������������
��3��500��ʱ��÷�Ӧ����ijʱ�̣�H2(g)��CO2(g)��CH3OH(g)��H2O(g)��Ũ�ȣ�mol/L���ֱ�Ϊ0.8��0.1��0.3��0.15�����ʱV��__________V������������������������������
��.һ�������£����ݻ�Ϊ2L���ܱ������г���lmolCO��2molH2�ϳɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
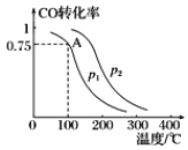
��1��p1__________p2�����������������������
��2���÷�Ӧ�ﵽƽ��ʱ����Ӧ��ת���ʵĹ�ϵ��CO____________H2�����������������������
��3����100��P1ʱ��ƽ�����õ�ʱ��Ϊ5min����ӿ�ʼ��ƽ�����ʱ����H2��ʾ������Ϊ_______________________��
��4���ü״��ϳɷ�Ӧ��A���ƽ�ⳣ��K��___________��