��Ŀ����
����Ŀ��ijѧϰС����ʵ�����о�SO2��Ba��NO3��2��Һ�ķ�Ӧ��
ʵ�飺��ʢ��2mL 0.1mol/L Ba��NO3��2��Һ���Թ��У�����ͨ��SO2���壬�Թ����а�ɫ����������Һ���Ϸ�����dz��ɫ��
̽��1����ɫ����������ԭ��
��1����ɫ������_________���ѧʽ����
��2��������ɫ����������ԭ��ͬѧ��Ϊ��NO3��������SO2����ͬѧ��Ϊ����Һ���ܽ��O2������SO2��
��֧�ּ�ͬѧ�۵��ʵ��֤����______________��
����ͬѧͨ������ʵ��֤�����Լ����Ʋ���ȷ�������ʵ�鷽����
ʵ����� | ʵ������ |
��2mL___mol��L ___��Һ���ѧʽ����ͨ��SO2 | ____________ |
̽��2��������SO2�Ĺ����У�NO3����O2������������Ҫ���á�
ʵ����� | ʵ������ |
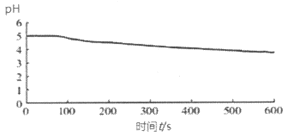
���ձ��м�������˵�0.1 mol��L��BaCl2��Һ25mL���ټ���25mLֲ���ͣ���ȴ�����£���pH�������ⶨ��ҺpH��ʱ�䣨t���ı仯���� |
ͼ1����BaCl2 ����������Һ��ͨ��SO2 |
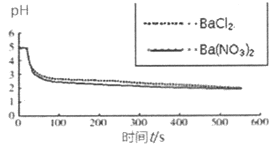
���ձ��зֱ����25mL 0.1 mol��L��BaCl2��Һ��Ba��NO3��2��Һ��ͨ��SO2����pH�������ֱ�ⶨ��ҺpH��ʱ�䣨t���仯�����ߡ� |
ͼ2���ֱ���BaCl2��Ba��NO3��2��Һ��ͨ��SO2 |
��3��ͼ1�������������£���BaCl2��Һ�г���ͨ��SO2����ҺpH�½�����Ϊ_________���÷���ʽ��ʾ����
��4��ͼ2��BaCl2��Һ�з�����Ӧ�����ӷ���ʽΪ___________��
��5����������ͼ����ó��Ľ�����_______________��
���𰸡�BaSO4 Һ���Ϸ�����dz��ɫ 0.1 mol/L BaCl2��Һ ���ɰ�ɫ���� SO2+H2O![]() H2SO3 ��H2SO3
H2SO3 ��H2SO3![]() HSO3��+H+ 2Ba2++2SO2+O2��2BaSO4+4H+ ����������������У���������Ҫ����
HSO3��+H+ 2Ba2++2SO2+O2��2BaSO4+4H+ ����������������У���������Ҫ����
��������
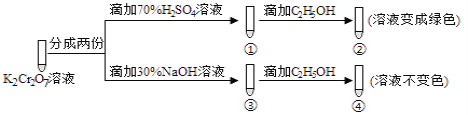
��1��SO2��Ba��NO3��2��Һ�ķ�Ӧ������Ԫ���غ��֪����ɫ����ӦΪBaSO4��
��2����NO3-������SO2�������������ԭ��һ��������һ�����������ٱ������ɶ���������ʹ��Һ���Ϸ�����dz��ɫ������֧�ּ�ͬѧ�۵��ʵ��֤����Һ���Ϸ�����dz��ɫ��
����ͬѧҪ֤�����Լ����Ʋ���ȷ��Ӧʹ�ñ����������Ӳ��������������������ſ������Աȣ����������Լ�Ϊ0.1mol/LBaCl2��Һ�����Կ����а�ɫ�������֣�
��3�������������£�������������ˮ���������ᣬ�������ʹ��pHֵ�½�����Ӧ�ķ���ʽΪSO2+H2O![]() H2SO3 ��H2SO3
H2SO3 ��H2SO3![]() HSO3��+H+��
HSO3��+H+��
��4��ͼ2��BaCl2��Һ��ͨ�����������ͼ1��������������ӣ�˵�������μ��˷�Ӧ�����������ᱵ�����ᣬ��Ӧ�����ӷ���ʽΪ2Ba2++2SO2+O2��2BaSO4+4H+��
��5���Ƚ�ͼ1��ͼ2��֪��������SO2�Ĺ����У�O2������Ҫ���ã���Ϊ��BaCl2��Һ�����������õ���O2����Ba��NO3��2��Һ�����������õ���O2��NO3-����ͼ2�У��ֱ���BaCl2��Ba��NO3��2��Һ��ͨ��SO2��pH�仯���ơ����Ƚӽ���
�ʴ�Ϊ��������SO2�Ĺ����У�O2������Ҫ���á�

����Ŀ����ͨ����н�������ʵ�飺
���� |
|
| |
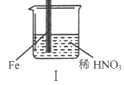
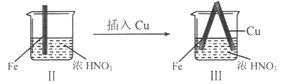
���� | Fe�������������ɫ���ݣ�Һ���Ϸ���Ϊ����ɫ | Fe���������������ɫ���ݺ�Ѹ��ֹͣ | Fe��Cu�Ӵ�����������������ɫ���� |
����˵������ȷ����
A. I����������ɫ�����ɫ�Ļ�ѧ����ʽ��2NO+O2��2NO2
B. II�е�����˵��Fe�����γ����ܵ������㣬��ֹFe��һ����Ӧ
C. �Ա�I��II������˵��ϡHNO3��������ǿ��ŨHNO3
D. ��Ԣ���������Fe��Cu֮�����ӵ����ƣ����ж�Fe�Ƿ�����
����Ŀ��A��B��C��D��E��F��G��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӣ�BԪ��ԭ�������������Ǵ�����������2����D�ǵؿ��к�������Ԫ�أ�E�Ļ�������ɫ��Ӧ�ǻ�ɫ��F��Gλ�����ڣ�G��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�ء�
���û�ѧ����ش�
��1��D�ļ������ӵĽṹʾ��ͼ��___________��
��2���õ���ʽ��ʾE2F���γɹ���____________��
��3��E��F��G����Ԫ�����γɵļ����ӣ��뾶�ɴ�С��˳����_________>_________>_________��
��4������ʵ�������Ӧ��ʵ�������У�����ȷ����_________������ĸ����
ѡ�� | ʵ����� | ʵ������ |
a | ��E����Ͷ�뵽CuSO4��Һ�� | ���ɴ�����ɫ���� |
b | ��AlCl3��Һ��ͨ�����C����̬�⻯�� | �����ɰ�ɫ������Ȼ������ܽ� |
c | ��G�ĵ���ͨ�뵽NaBr��Һ�г�ַ�Ӧ�������Ȼ�̼�������� | �²���Һ��Ϊ��ɫ |
d | ��B�����������ͨ�뵽Na2SiO3��Һ�� | ���ɰ�ɫ���� |
��5��д��A��B�γɵ�10���ӷ��ӵĻ�ѧʽ_________����������G�ĵ����ڹ����·�Ӧ��һ��ʱ�������װ��ʾ��ͼ������ȷ��ӳʵ���������_________������ĸ����

��6����Fe��Cu�Ļ�����У�����һ������C������������Ӧ��ˮ�����ϡ��Һ����ַ�Ӧ��ʣ�����m1 g���������м���һ������ϡ���ᣬ��ַ�Ӧ��ʣ�����m2 g������˵����ȷ����_________������ĸ����
a m1����m2 b m1����m2
c ʣ����Һ��һ����Fe3+ d ʣ�������һ����Cu