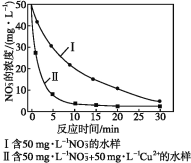
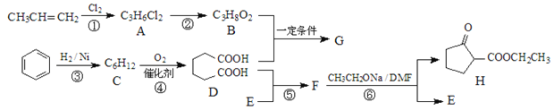
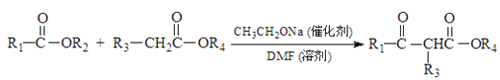
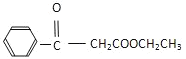ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩœ¬±μ «‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷Θ§’κΕ‘±μ÷–ΒΡΔΌΓΪΔβ÷÷‘ΣΥΊΘ§«κ”ΟΜ·―ß”Ο”οΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Ήε ÷ήΤΎ | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
2 | Δό | ΔΏ | Δύ | |||||
3 | ΔΌ | Δέ | Δί | Δα | Δβ | |||
4 | ΔΎ | Δή |
Θ®1Θ©‘ΎΔέΓΪΔΏ‘ΣΥΊ÷–Θ§‘≠Ή”ΑκΨΕΉν¥σΒΡ «_____Θ®Χν‘ΣΥΊΖϊΚ≈Θ©ΘΜΔβ‘ΣΥΊΖϊΚ≈ΈΣ_____ΘΜ
Θ®2Θ©ΔΏ‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·Έο”κΤδ«βΜ·ΈοΡή…ζ≥…―Έ MΘ§M ÷–Κ§”–ΒΡΜ·―ßΦϋάύ–Ά”–_____ΘΜ
Θ®3Θ©–¥≥ω‘ΣΥΊΔΌΚΆΔύΒΡΒΞ÷ ‘ΎΦ”»»ΧθΦΰœ¬Ζ¥”Π…ζ≥…ΒΡΜ·ΚœΈοΒΡΒγΉ” ΫΘΚ_____ΓΘ
Θ®4Θ©ΔέΓΔΔίΓΔΔΏΓΔΔύΥυ–Έ≥…ΒΡάκΉ”Θ§ΤδΑκΨΕ”…–ΓΒΫ¥σΒΡΥ≥–ρ «_____Θ®ΧνάκΉ”ΖϊΚ≈Θ©ΓΘ
Θ®5Θ©ΔΌΓΪΔα÷–‘ΣΥΊΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·Έο÷–Υα–‘Ήν«ΩΒΡ «_____Θ®ΧνΈο÷ Μ·―ß ΫΘ©ΘΜ≥ ΝΫ–‘ΒΡ«β―θΜ·Έο «_____Θ®ΧνΈο÷ Μ·―ß ΫΘ©,ΗΟΜ·ΚœΈο”κNaOH »ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ_________ΓΘ
Θ®6Θ©”ΟΒγΉ” Ϋ±μ Ψ‘ΣΥΊΔέ”κΔα–Έ≥…Μ·ΚœΈοΒΡΙΐ≥Χ_____ΓΘ
ΓΨ¥πΑΗΓΩCa Ar άκΉ”ΦϋΚΆΙ≤ΦέΦϋ ![]() Al3+ΘΦMg2+ΘΦO2ΘΦN3 HClO4 Al(OH)3 Al(OH)3 + OHΘ≠ = AlO2Θ≠ + 2H2O
Al3+ΘΦMg2+ΘΦO2ΘΦN3 HClO4 Al(OH)3 Al(OH)3 + OHΘ≠ = AlO2Θ≠ + 2H2O ![]()
ΓΨΫβΈωΓΩ
ΗυΨί÷ήΤΎ±μΒΟ≥ωΔΌΓΪΔβ‘ΣΥΊΖ÷±πΈΣNaΓΔKΓΔMgΓΔCaΓΔAlΓΔCΓΔNΓΔOΓΔClΓΔArΓΘΨί¥ΥΫβ¥πΓΘ
Θ®1Θ©ΗυΨί≤ψΕύΨΕ¥σΩ…÷Σ‘ΎΔέΓΪΔΏ‘ΣΥΊ÷–Θ§‘≠Ή”ΑκΨΕΉν¥σΒΡ «CaΘΜΔβ‘ΣΥΊΖϊΚ≈ΈΣArΘΜΙ ¥πΑΗΈΣΘΚCaΘΜArΓΘ
Θ®2Θ©ΔΏ‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·ΈοΈΣHNO3”κΤδ«βΜ·ΈοNH3Ρή…ζ≥…―ΈM(NH4NO3)Θ§M÷–Κ§”–ΒΡΜ·―ßΦϋάύ–Ά”–άκΉ”ΦϋΚΆΙ≤ΦέΦϋΘΜΙ ¥πΑΗΈΣΘΚάκΉ”ΦϋΚΆΙ≤ΦέΦϋΓΘ
Θ®3Θ©‘ΣΥΊΔΌΚΆΔύΒΡΒΞ÷ ‘ΎΦ”»»ΧθΦΰœ¬Ζ¥”Π…ζ≥…ΒΡΜ·ΚœΈοΈΣNa2O2Θ§ΤδΒγΉ” ΫΘΚ![]() ΘΜΙ ¥πΑΗΈΣΘΚ
ΘΜΙ ¥πΑΗΈΣΘΚ![]() ΓΘ
ΓΘ
Θ®4Θ©ΔέΓΔΔίΓΔΔΏΓΔΔύΥυ–Έ≥…ΒΡάκΉ”Θ§ΒγΉ”≤ψΫαΙΙœύΆ§Θ§ΗυΨίΚΥΕύΨΕ–Γ‘≠‘ρΒΟΒΫΑκΨΕ”…–ΓΒΫ¥σΒΡΥ≥–ρ «Al3+ΘΦMg2+ΘΦO2ΘΦN3ΘΜΙ ¥πΑΗΈΣΘΚAl3+ΘΦMg2+ΘΦO2ΘΦN3ΓΘ
Θ®5Θ©Ζ«Ϋπ τ‘Ϋ«ΩΘ§ΤδΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·ΈοΒΡΥα–‘‘Ϋ«ΩΘ§“ρ¥ΥΔΌΓΪΔα÷–‘ΣΥΊΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·Έο÷–Υα–‘Ήν«ΩΒΡ «HClO4ΘΜ≥ ΝΫ–‘ΒΡ«β―θΜ·Έο «Al(OH)3Θ§ΗΟΜ·ΚœΈο”κNaOH »ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣAl(OH)3 + OHΘ≠ = AlO2Θ≠ + 2H2OΘΜΙ ¥πΑΗΈΣΘΚHClO4ΘΜAl(OH)3ΘΜAl(OH)3 + OHΘ≠ = AlO2Θ≠ + 2H2OΓΘ
Θ®6Θ©ΟΨΚΆ¬»ΤχΖ¥”Π…ζ≥…¬»Μ·ΟΨΘ§”ΟΒγΉ” Ϋ±μ Ψ–Έ≥…¬»Μ·ΟΨΒΡ–Έ≥…Ιΐ≥ΧΈΣΘΚ![]() ΘΜΙ ¥πΑΗΈΣΘΚ
ΘΜΙ ¥πΑΗΈΣΘΚ![]() ΓΘ
ΓΘ

 ΩΎΥψΧβΩ®±±Ψ©ΗΨ≈°ΕυΆ·≥ωΑφ…γœΒΝ–¥πΑΗ
ΩΎΥψΧβΩ®±±Ψ©ΗΨ≈°ΕυΆ·≥ωΑφ…γœΒΝ–¥πΑΗ