��Ŀ����
����Ŀ�����ݻ�Ϊ![]() ���ܱ������г���
���ܱ������г���![]() �����
�����![]() ���壬��һ�������·������·�Ӧ��
���壬��һ�������·������·�Ӧ��![]() ����
����![]() ��ﵽƽ�⣬���C�����Ũ��Ϊ
��ﵽƽ�⣬���C�����Ũ��Ϊ![]() ������˵������ȷ����
������˵������ȷ����![]()
![]()
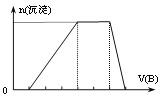
��������A��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ![]()
��������B��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ![]()
��ƽ��ʱ����A��B��ת�������
��ƽ��ʱ����B��Ũ��Ϊ![]()
�������������䣬���������ټ���![]() ���壬�ﵽ��ƽ��ʱ��C�������������
���壬�ﵽ��ƽ��ʱ��C�������������
A.�٢ڢ�B.�٢���C.�ۢ���D.�ڢۢ�
���𰸡�B
��������
��C��Ũ�ȱ仯Ϊ0.6mol/L������![]() ������v��C������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��A����
������v��C������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��A����
��C��Ũ�ȱ仯Ϊ0.6mol/L������![]() ������v��C������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��B����
������v��C������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��B����
��A��B��ѧ������֮��Ϊ2��1���μӷ�Ӧ��A��B�����ʵ���֮��Ϊ2��1��A��B����ʼ���ʵ���֮��Ϊ2��1��ƽ��ʱ����A��B��ת������ȣ�
�ܸ���C��Ũ�ȱ仯������B��Ũ�ȱ仯����ƽ��ʱ����B��Ũ�ȵ���B��ʼŨ�ȼ�ȥB��Ũ�ȱ仯����
��ԭƽ���뿪ʼ����3molC�ǵ�Ч�ģ���ԭƽ��״̬����1molC�����Ե�ЧΪ��ʼ����4molC���÷�Ӧ��Ӧǰ����������ʵ����仯���ں��º�������£�ѹǿ����ƽ��״̬��ͬ��
��C��Ũ�ȱ仯Ϊ0.6mol/L,����![]() ,����֮�ȵ��ڻ�ѧ������֮��,����
,����֮�ȵ��ڻ�ѧ������֮��,����![]() ���ʢ���ȷ��
���ʢ���ȷ��
���ɢ�֪,v(C)=0.3mol/(LS),����֮�ȵ��ڻ�ѧ������֮��,����![]() ���ʢڴ���
���ʢڴ���
��A. B��ѧ������֮��Ϊ2:1���μӷ�Ӧ��A. B�����ʵ���֮��Ϊ2:1��A. B����ʼ���ʵ���֮��Ϊ2:1��ƽ��ʱ����A��B��ת������ȣ��ʢ���ȷ��
�ܡ�c(C)=0.6mol/L,����![]() ��֪,
��֪, ![]() ������Bƽ��Ũ��Ϊ
������Bƽ��Ũ��Ϊ![]() ���ʢܴ���
���ʢܴ���
��ԭƽ���뿪ʼ����3molC�ǵ�Ч�ģ���ԭƽ��״̬����1molC�����Ե�ЧΪ��ʼ����4molC���÷�Ӧ��Ӧǰ����������ʵ����仯���ں��º�������£�ѹǿ����Ӱ��ƽ���ƶ�������3molC�����4molC�������ƽ��״̬��ͬ���������������ټ���1molC���壬�ﵽ��ƽ��ʱ��C������������䣬�ʢ���ȷ��
�٢ۢ���ȷ����ѡB��