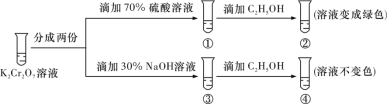
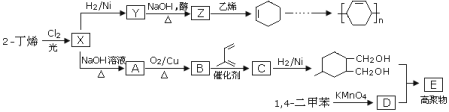
��Ŀ����
����Ŀ������˵����ȷ���ǣ� ��
A.101kPaʱ��2H2(g)+O2(g)=2H2O(l) ��H=-572kJ��mol-1����H2��ȼ������H=-572kJ��mol-1
B.һ�������·�����Ӧ��N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4kJ��mol-1���������½�1.5mol H2����N2��ַ�Ӧ���ų�����46.2kJ
2NH3(g) ��H=-92.4kJ��mol-1���������½�1.5mol H2����N2��ַ�Ӧ���ų�����46.2kJ
C.�������������������������ֱ���ȫȼ�գ����߷ų�������
D.��֪��2C(s)+2O2(g)=2CO2(g) ��H1��2C(s)+O2(g)=2CO(g) ��H2������H1����H2
���𰸡�D
��������
A��ȼ������1mol��ȼ����ȫȼ�������ȶ���������ʱ�ų�����������572kJ��2mol����ȼ�շų���������A����
B��N2(g)+3H2(g)![]() 2NH3(g)�ǿ��淴Ӧ�����ܽ��е��ף��ų���������С��46.2kJ��B����
2NH3(g)�ǿ��淴Ӧ�����ܽ��е��ף��ų���������С��46.2kJ��B����
C����̬��ȹ�̬�������ߣ�ȼ�շ��ȶ࣬C����
D��������̼��ȫȼ�����ɶ�����̼�ų����������ڲ���ȫȼ������CO�ų�������������HΪ��ֵ��������H1����H2��D��ȷ��
��ѡD��

����Ŀ��Ϊ�˲ⶨ���ᾧ��H2C2O4��xH2O�е�xֵ��ijʵ��С�����ʵ�飬�������£�
�ٳ�ȡ1.260 g���ᾧ�壬���100 mL��Һ��
��ȡ25.00 mL��H2C2O4��Һ������ƿ�ڣ��ټ�������ϡ���ᡣ
����Ũ��Ϊ0.1000 mol/L��KMnO4��Һ�ζ�H2C2O4��Һ����__________________ʱ���ζ�������
�ܼ�¼���ݣ��ظ�ʵ�顣���������磺
ʵ����� | V(KMnO4��Һ) | |
�ζ�ǰ�̶�/mL | �ζ���̶�/mL | |
1 | 0.10 | 10.00 |
2 | 1.10 | 11.10 |
3 | 1.50 | 11.50 |
�ش��������⣺
(1)�������ʹ���ձ�����Ͳ������������ȱ�ٵIJ�������Ϊ______________(������)������۵ζ������У�ʢװKMnO4��Һ������Ϊ_______________(������)��
(2)�÷�Ӧԭ���Ļ�ѧ����ʽΪ_____________________________________________��
(3)�뽫����۲�������_____________________________________________________��
(4)�������ݣ�����H2C2O4��Һ�����ʵ���Ũ��Ϊ_________mol/L��x=________��
(5)���ζ��յ����ʱ����KMnO4��ҺҺ�棬��xֵ��_________(�ƫ��ƫС������Ӱ�족)��
����Ŀ��Ϊ�ⶨCO2����Է���������ijʵ��С����λͬѧѡ�ú�NaHCO3����Ʒ(������Ϊm1g)�������������Լ�����������������ʵ�顣���������գ�
����������ȷ��CO2��������װ������ͼ��
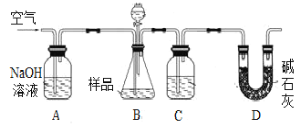
(1)B�з�Ӧ�Ļ�ѧ����ʽΪ____________________________________________________��
(2)ʵ���г�������ͨ�������������֮һ�ǰ����ɵ�CO2ȫ���������װ���У�ʹ֮��ȫ�����գ���������Ϊ___________________________________________________________��
(3)������߲ⶨ��ȷ�ȵĴ�ʩ��___________��
a����B�ڼ�����֮ǰ���ž�װ���ڵ�CO2����
b����B�ڵμ���ʱ���˹���
c����B��C֮������ʢ�б���NaHCO3��Һ��ϴ��װ��
d����D������ʢ�м�ʯ�ҵĸ����
���õζ���ȷ��CO2�����ʵ���������Ʒ���Ƴ�100mL��Һ������ȡ��20.00 mL����c mol��L��1������ζ�(������ָʾ��)����______________________________________________________ʱ��ֹͣ�ζ���ƽ�вⶨ���Σ��й�ʵ�����ݼ�¼���±���m1 g��Ʒ����CO2�����ʵ���Ϊ_____________��
ʵ���� | ����Һ��� (mL) | �����������(mL) | |
������ | ĩ���� | ||
1 | 20.00 | 0.00 | 25.02 |
2 | 20.00 | 0.20 | 28.80 |
3 | 20.00 | 1.30 | 26.28 |
�������������ȷ��CO2�������װ����ͼ��ʾ��

(4)Ϊ�˼�Сʵ�����������м����Һ��XΪ___________________��Һ��
(5)����װ�����������ã�����ƽ�ӣ�����õ���CO2�����������ȻƫС����ԭ�������____________________________________________________________________________��
(6)ȷ��CO2����Է���������ѡ��___________________(��������������������������������д)��ʵ������Ϊ��ѡ�
����Ŀ��ij�¶���2L�ܱ������У�3��������ʼ״̬��ƽ��״̬ʱ�����ʵ�����n�����±���ʾ������˵����ȷ���ǣ� ��
X | Y | W | |
n����ʼ״̬��/mol | 2 | 1 | 0 |
n��ƽ��״̬��/mol | 1 | 0.5 | 1.5 |
A.���¶��´�ƽ�������ѹǿƽ�ⲻ�ƶ�
B.�÷�Ӧ����ʽ�ɱ�ʾΪ��X+2Y=3W
C.�����¶ȣ���W�����������С����˷�Ӧ��H��0
D.���º���ʱ������X�����ʵ�����ƽ���������ƶ���X��ת�������