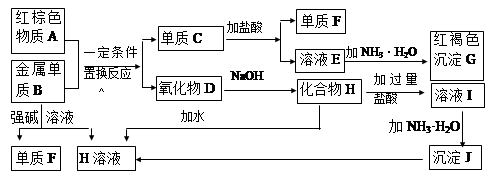��Ŀ����
A+B��X+Y+H2O��δ��ƽ����Ӧ������ȥ������ѧ������Ӧ�Ļ�ѧ����ʽ������A��B�����ʵ���֮��Ϊ1��4����ش�
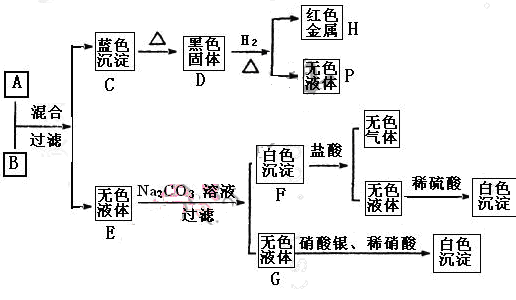
��1����Y�ǻ���ɫ���壬��÷�Ӧ�����ӷ���ʽ�� _____________________________����Y����������SO2��ֻ�Ϻ�ͨ��Ʒ����Һ��δ������ɫ��ԭ����__________________________________________�����û�ѧ����ʽ����˵������
��2����AΪ�ǽ������ʣ���������ԭ�Ӻ��������������Ǵ�����������2����B����
ҺΪijŨ�ᣬ��Ӧ���õ���������Ļ�ѧʽΪ ��
��3����AΪ�������ʣ�������A��B��Ũ��Һ�С��ۻ���������A������X��Һ�У�
�� AԪ�ص�Ԫ�ط���Ϊ ����A��B�ķ�Ӧ���������뻹ԭ�������ʵ���֮���� ��
�� ����a mol X����Һ�ܽ���һ����A��ʱ��Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X�����ʵ���Ϊ mol���ú�a����ʽ��ʾ����Ϊ��֤A��B��ַ�Ӧ���������õ���Һ��ͬʱ�����������ֽ��������ӣ����ʱB��A��������Ӧ�����ȡֵ��Χ�� ��
��1����Y�ǻ���ɫ���壬��÷�Ӧ�����ӷ���ʽ�� _____________________________����Y����������SO2��ֻ�Ϻ�ͨ��Ʒ����Һ��δ������ɫ��ԭ����__________________________________________�����û�ѧ����ʽ����˵������
��2����AΪ�ǽ������ʣ���������ԭ�Ӻ��������������Ǵ�����������2����B����
ҺΪijŨ�ᣬ��Ӧ���õ���������Ļ�ѧʽΪ ��
��3����AΪ�������ʣ�������A��B��Ũ��Һ�С��ۻ���������A������X��Һ�У�
�� AԪ�ص�Ԫ�ط���Ϊ ����A��B�ķ�Ӧ���������뻹ԭ�������ʵ���֮���� ��
�� ����a mol X����Һ�ܽ���һ����A��ʱ��Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X�����ʵ���Ϊ mol���ú�a����ʽ��ʾ����Ϊ��֤A��B��ַ�Ӧ���������õ���Һ��ͬʱ�����������ֽ��������ӣ����ʱB��A��������Ӧ�����ȡֵ��Χ�� ��
��1��MnO2+4Cl��+2H��
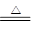 Mn2��+Cl2��+2H2O ��2�֣� SO2 + Cl2 + 2H2O = H2SO4 + 2HCl ��2�֣�
Mn2��+Cl2��+2H2O ��2�֣� SO2 + Cl2 + 2H2O = H2SO4 + 2HCl ��2�֣���2��CO2��1�֣�
��3����Fe��1�֣� 1��1 ��1�֣� ��0.4a��1�� �� 3/1 < m(B)/m(A) < 9/2 ��2�֣�
�����������1����ѧ��ѧ�л���ɫ�������ָ������MnO2+4Cl��+2H��
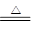 Mn2��+Cl2��+2H2O������������������SO2��ֻ�Ϻ�ͨ��Ʒ����Һ��Һ����ɫ������Ϊ���߷�Ӧ������������ᣬʧȥƯ���ԡ�
Mn2��+Cl2��+2H2O������������������SO2��ֻ�Ϻ�ͨ��Ʒ����Һ��Һ����ɫ������Ϊ���߷�Ӧ������������ᣬʧȥƯ���ԡ� SO2 + Cl2 + 2H2O = H2SO4 + 2HCl ��2�֣�
��2��ԭ�Ӻ��������������Ǵ�����������2��������A��C��A��B�����ʵ���֮��Ϊ1��4������Bһ����Ũ���ᡣ��˷�Ӧ���õ���������Ļ�ѧʽΪ��CO2��1�֣�
��3���ٽ������ʳ����·������ۻ�����ֻ��������������ֻ�������������������ӵ���Һ�����AԪ�ص�Ԫ�ط���Ϊ��Fe��Fe��4HNO3��Fe(NO3)3��NO����2H2O ��A��B�ķ�Ӧ���������뻹ԭ�������ʵ���֮����1��1
�� Fe �� 2Fe3�� �� 3Fe2��
��ʼ�� amol 0mol
ת���� bmol 1.5bmol
���գ� (a��b)mol 1.5bmol
��������(a��b)mol��1.5bmol
��֮�ã�b��0.4a
Fe��4HNO3(ϡ)��Fe(NO3)3��NO����2H2O��3Fe��8HNO3(ϡ)��3Fe(NO3)2��2NO����4H2O
56 4��63 3��56 8��63
����B��A��������Ӧ�����ȡֵ��Χ�ǣ�3/1 < m(B)/m(A) < 9/2 ��2�֣�

��ϰ��ϵ�д�
�����Ŀ