��Ŀ����
��Ȼ�л�����A�Ƿ����廯�������̼������������������������2������ȫȼ�պ���õ����ʵ���֮��Ϊ2�U1��CO2��H2O��A���м��������ԣ�������HI���õõ�B���ṹ������ʾA����������ͬ�����Ŷ�B�����ڶ�λ����ͬ�����ţ�A�������������B�������������
��֪ R�CO�CR�� +HI��R�CO�CH + R��CI
(1 ) A�Ļ�ѧʽΪ
(2) д��A��B�Ľṹ��ʽ��B�б������� ,
(3) ��A������ͬ�����ŵ�λ���칹���� �֣���������
��B������ͬ�����ŵ�λ���칹���� �֣���������
(4) A����һ���칹��C�������ԣ�������NaOH��1�U3��Ӧ��
д��C��NaOH��Ӧ�Ļ�ѧ����ʽ ��
��֪ R�CO�CR�� +HI��R�CO�CH + R��CI
(1 ) A�Ļ�ѧʽΪ
(2) д��A��B�Ľṹ��ʽ��B�б������� ,
(3) ��A������ͬ�����ŵ�λ���칹���� �֣���������
��B������ͬ�����ŵ�λ���칹���� �֣���������
(4) A����һ���칹��C�������ԣ�������NaOH��1�U3��Ӧ��
д��C��NaOH��Ӧ�Ļ�ѧ����ʽ ��
(1)C8H8O3��2�֣�
(2)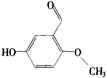 ��2�֣�
��2�֣�  ��2�֣�
��2�֣�
(3)A��10�� B��6�֣���2�֣�
(4) ��OCOOCH3��3NaOH��
��OCOOCH3��3NaOH�� ��ONa��Na2CO3��CH3OH��H2O��2�֣�
��ONa��Na2CO3��CH3OH��H2O��2�֣�
(2)
 ��2�֣�
��2�֣�  ��2�֣�
��2�֣�(3)A��10�� B��6�֣���2�֣�
(4)
 ��OCOOCH3��3NaOH��
��OCOOCH3��3NaOH�� ��ONa��Na2CO3��CH3OH��H2O��2�֣�
��ONa��Na2CO3��CH3OH��H2O��2�֣���A�ķ���ʽΪ��CxHyOz��������֪12x��16z=2:1�����Ƴ�x��z=8��3��
��ȼ�պ�����CO2��H2O=1��1������Ƴ�x��y=1��1��
����A�����ʽΪ��C8H8O3
�������֪��A�����д��ڱ��������ǻ����ѽṹ��������Ҫ��һ��˫���������Ͷ�Ϊ5����
(2)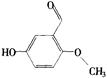 ��2�֣�
��2�֣�  ��2�֣�
��2�֣�
��3�����ö�����һ�������ɵó�A�����ϵ�ͬ���칹��Ϊ10�֣�BΪ6�֣�ע�ⲻҪ�ظ���
(4) ��OCOOCH3��3NaOH��
��OCOOCH3��3NaOH�� ��ONa��Na2CO3��CH3OH��H2O��2�֣�
��ONa��Na2CO3��CH3OH��H2O��2�֣�
��ȼ�պ�����CO2��H2O=1��1������Ƴ�x��y=1��1��
����A�����ʽΪ��C8H8O3
�������֪��A�����д��ڱ��������ǻ����ѽṹ��������Ҫ��һ��˫���������Ͷ�Ϊ5����
(2)
 ��2�֣�
��2�֣�  ��2�֣�
��2�֣���3�����ö�����һ�������ɵó�A�����ϵ�ͬ���칹��Ϊ10�֣�BΪ6�֣�ע�ⲻҪ�ظ���
(4)
 ��OCOOCH3��3NaOH��
��OCOOCH3��3NaOH�� ��ONa��Na2CO3��CH3OH��H2O��2�֣�
��ONa��Na2CO3��CH3OH��H2O��2�֣�
��ϰ��ϵ�д�
�����Ŀ