��Ŀ����
��8�֣�A��һ�ֺ�̼���⡢������Ԫ�ص��л��������֪��A��Է�������Ϊ144������̼����������Ϊ50%��A����ʹ��ˮ��ɫ��A������ˮ���������������¿ɷ���ˮ�ⷴӦ���õ�B�ͼ״���B��̼���⡢��ԭ�Ӹ�����Ϊ1��1��1��������û��֧������������������Һ������Ӧ��
��1��A���Է����ķ�Ӧ�� ��ѡ���ţ�
�ټӳɷ�Ӧ ��������Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��B�������������ŵ������� �� ��
��3����B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ�� ________________________________________________��
��4����B��ȡA�Ļ�ѧ����ʽ�ǣ� ��
��1��A���Է����ķ�Ӧ�� ��ѡ���ţ�
�ټӳɷ�Ӧ ��������Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��B�������������ŵ������� �� ��
��3����B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ�� ________________________________________________��
��4����B��ȡA�Ļ�ѧ����ʽ�ǣ� ��
���Ţ٢ۢ� ��̼̼˫�� �Ȼ� �� CH2��C(COOH)2
�� HOOCCH��CHCOOH + 2CH3OH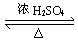 CH3OOCCH��CHCOOCH3 + 2H2O
CH3OOCCH��CHCOOCH3 + 2H2O
�� HOOCCH��CHCOOH + 2CH3OH
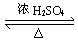 CH3OOCCH��CHCOOCH3 + 2H2O
CH3OOCCH��CHCOOCH3 + 2H2O����A�����ʿ�֪A�к���̼̼�����ͼ�������������̼ԭ�ӵĸ����� ������ˮ�����ɼ״���������A��ֻ��1����������B�ķ���ʽ����C5H5O5,���Լ����Dz��ܳ����ģ�����A�к���2��������ˮ������2���Ӽ״������B�ķ���ʽΪC4H4O4,����ΪB��û��֧��������B�Ľṹ��ʽΪHOOCCH��CHCOOH����A�Ľṹ��ʽΪCH3OOCCH��CHCOOCH3��ͨ���ı��Ȼ���λ�ü��õ���B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪCH2��C(COOH)2��
������ˮ�����ɼ״���������A��ֻ��1����������B�ķ���ʽ����C5H5O5,���Լ����Dz��ܳ����ģ�����A�к���2��������ˮ������2���Ӽ״������B�ķ���ʽΪC4H4O4,����ΪB��û��֧��������B�Ľṹ��ʽΪHOOCCH��CHCOOH����A�Ľṹ��ʽΪCH3OOCCH��CHCOOCH3��ͨ���ı��Ȼ���λ�ü��õ���B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪCH2��C(COOH)2��
 ������ˮ�����ɼ״���������A��ֻ��1����������B�ķ���ʽ����C5H5O5,���Լ����Dz��ܳ����ģ�����A�к���2��������ˮ������2���Ӽ״������B�ķ���ʽΪC4H4O4,����ΪB��û��֧��������B�Ľṹ��ʽΪHOOCCH��CHCOOH����A�Ľṹ��ʽΪCH3OOCCH��CHCOOCH3��ͨ���ı��Ȼ���λ�ü��õ���B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪCH2��C(COOH)2��
������ˮ�����ɼ״���������A��ֻ��1����������B�ķ���ʽ����C5H5O5,���Լ����Dz��ܳ����ģ�����A�к���2��������ˮ������2���Ӽ״������B�ķ���ʽΪC4H4O4,����ΪB��û��֧��������B�Ľṹ��ʽΪHOOCCH��CHCOOH����A�Ľṹ��ʽΪCH3OOCCH��CHCOOCH3��ͨ���ı��Ȼ���λ�ü��õ���B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪCH2��C(COOH)2��
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
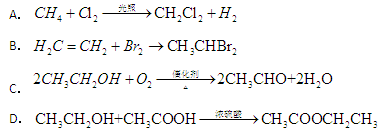
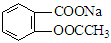 ת��Ϊ
ת��Ϊ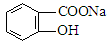 ����Ϊ ( )
����Ϊ ( )