��Ŀ����
12��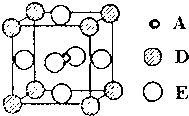 A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ������������Aԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��C�Ļ�̬ԭ��2p�ܼ�����2��δ�ɶԵ��ӣ�C2-������D2+���Ӿ�����ͬ�ĵ��Ӳ�ṹ��E�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽΪ3d84s2��
A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ������������Aԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��C�Ļ�̬ԭ��2p�ܼ�����2��δ�ɶԵ��ӣ�C2-������D2+���Ӿ�����ͬ�ĵ��Ӳ�ṹ��E�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽΪ3d84s2����ش��������⣺
��1��E��Ԫ�����ڱ��е������ڣ��ڢ������Ԫ�أ�
��2��A��B��C����Ԫ���У��縺��������O����Ԫ�ط��ţ���
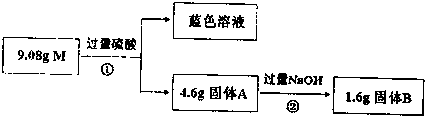
��3��A��C����Ԫ�ؿ��γɷ���ʽΪAC2�Ĺ��ۻ��������ӵ�����ṹ��ֱ���ͣ���A��B��C�γɵ�����CAB-��AC2��Ϊ�ȵ����壬CAB-��Aԭ�ӵ��ӻ���ʽ��sp��
��4����A��D��E����Ԫ���γɵ�һ�־�����г����ԣ�
�侧���ṹ����ͼ��ʾ���þ���Ļ�ѧʽΪNi3MgC��
�þ�����ÿ��Dԭ����Χ���������Eԭ����12����
��5��E�ĵ��ʷ�ĩ��AC�����������ȣ�������ɫ�ӷ���Һ������E��AC��4��E��AC��4�������幹�ͣ���423Kʱ��E��AC��4�ֽ�Ϊ����E��AC���Ӷ��Ƶøߴ��ȵĵ���E��ĩ����E��AC��4�ľ��������Ƿ��Ӿ��壻E��AC��4�����ڢڢۣ�ѡ����ţ���
��ˮ �����Ȼ�̼ �۱� ����������Һ��
���� A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ������������Aԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��AΪC��C�Ļ�̬ԭ��2p�ܼ�����2��δ�ɶԵ��ӣ�C2-������D2+���Ӿ�����ͬ�ĵ��Ӳ�ṹ��˵��C�������6�����ӣ�����CΪO����DΪMg��E�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽΪ3d84s2����EΪNi���ݴ˴��⣮
��� �⣺A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ������������Aԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��AΪC��C�Ļ�̬ԭ��2p�ܼ�����2��δ�ɶԵ��ӣ�C2-������D2+���Ӿ�����ͬ�ĵ��Ӳ�ṹ��˵��C�������6�����ӣ�����CΪO����DΪMg��E�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽΪ3d84s2����EΪNi��
��1��EΪNi��λ�����ڱ��е������ڣ��ڢ����壬
�ʴ�Ϊ���ģ�����
��2��A��B��C����Ԫ����O�ķǽ�������ǿ���ǽ�����Խǿ�縺��Խǿ����O�ĵ縺����ǿ��
�ʴ�Ϊ��O��
��3��A��C����Ԫ�ؿ��γɷ���ʽΪCO2��̼ԭ����������ԭ��������û�йµ��Ӷԣ���������ӵ�����ṹ��ֱ���ͣ�CO2��CNO-��Ϊ�ȵ����壬�ȵ�������ӻ���ʽ��ͬ����֪CO2Ϊsp�ӻ�����CNO-Ҳ��sp�ӻ����ʴ�Ϊ��ֱ���ͣ�sp��
��4�����ݾ����ṹͼ�����þ�̯����֪��������̼ԭ����Ϊ1��þԭ����Ϊ$8��\frac{1}{8}$=1����ԭ����Ϊ$6��\frac{1}{2}$=3�����Ծ���Ļ�ѧʽΪNi3MgC�����ݾ����ṹͼ��֪��ÿ��Mgԭ����Χ���������Niԭ�ӷֲ��ھ�����þԭ�ӵľ�������������ϣ���12����
�ʴ�Ϊ��Ni3MgC��12��
��5��Ni��CO��4Ϊ��ɫ�ӷ���Һ̬���ʣ��۷е�ϵͣ���Ϊ���Ӿ��壬Ni��CO��4�������幹�ͣ�Ϊ�Ǽ��Է��ӣ�������������ԭ�������������Ȼ�̼��������ѡ�ڢۣ�
�ʴ�Ϊ�����Ӿ��壻�ڢۣ�
���� ���⿼�������ʽṹ�ƶ��⣬���õ����Ų����й�֪ʶ�����ƶϣ�ͬʱ�������ӻ����ȵ����壬������ѧʽ������ȣ��е��Ѷȣ�

| A�� | �٢ۢܢޢ� | B�� | ����ڶ��� | C�� | �٢ܢޢ� | D�� | �٢ۢܢ� |
| A�� | �÷�Ӧ�����û���Ӧ | |
| B�� | �÷�Ӧ������KClO3��ǿ������ | |
| C�� | �������뻹ԭ�������ʵ���֮��Ϊ1��6 | |
| D�� | �÷�Ӧ����5mo1����ת��ʱ����һ������67.2L Cl2 |
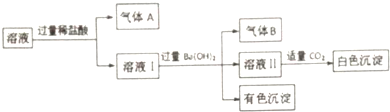
| A�� | ����Aһ��û��CO2������Bһ����NH3 | |
| B�� | ��ɫ����һ���ǻ���� | |
| C�� | Na+���ܴ����ڸ���Һ�� | |
| D�� | ��ɫ������һ��û��Al��OH��3 |
| A�� | H2O2+SO2�TH2SO4 | B�� | I2+2Fe2+�T2I+2Fe3+ | ||
| C�� | 2Fe3++SO2+2H2O�T2Fe2++SO42-+4H+ | D�� | SO2+2H2O+I2�TH2SO4+2HI |
| A�� | ���³�ѹ�£�17g����-14CH3��������������Ϊ9NA | |
| B�� | 42.0 g��ϩ�ͱ�ϩ�Ļ�������к��е�̼ԭ����Ϊ3NA | |
| C�� | ��״���£�11.2L���к��е�̼̼˫����Ϊ1.5NA | |
| D�� | 5.6g����0.1mol�����ڵ�ȼ�����³�ַ�Ӧ��ת�Ƶĵ�����Ϊ0.3NA |
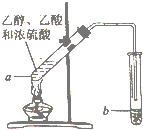 ����ͼ��ʾ��ʵ�װ���Ʊ�����������������ش��й����⣺
����ͼ��ʾ��ʵ�װ���Ʊ�����������������ش��й����⣺ CH3COOC2H5+H2O����Ӧ������������Ӧ����ȡ����Ӧ����
CH3COOC2H5+H2O����Ӧ������������Ӧ����ȡ����Ӧ����