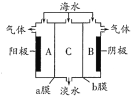
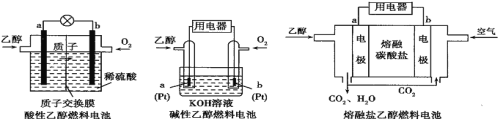
��Ŀ����
����Ŀ�����⻯��(NaAlH4)���л��ϳɵ�һ����Ҫ��ԭ����һ����Ʊ������ǽ�AlC13�����л��ܼ����ٰ�������Һ�μӵ�NaH��ĩ�ϣ����Ƶ����⻯�ơ�ʵ��Ҫ���װ�����£��ش��������⣺
(1)��ȡ���⻯��Ҫ�ڷ�ˮ��Һ�н��У���Ҫԭ����_______(�û�ѧ����ʽ��ʾ)��������װ����ȡ�������⻯��(�������ﴦ��)��Ϊ��������__________��

(2)����ԭ��A1C13����ȡ��ij��ȤС�����������װ�ã�
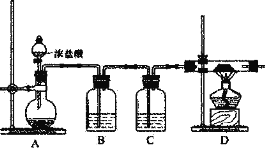
�����Ӻ�װ�ú�Ӧ���еĵ�һ��������_________��Ϊ��֤����Ĵ�����Ӧ��D��__________(������)�ټ��Ⱦƾ��ơ�
��Bװ����ʢװ����NaCl��Һ��ʵ�鿪ʼ��B�в�����������_________��ѡ��NaCl��Һ��������_____��
��Cװ�õ�������_________������Cװ�ã���Ԥ��ʵ���D���������A1C13����ܻ�����_________��(�ѧʽ)
������װ�ô��ڵ�����ȱ����_________��
���𰸡�NaAlH4+2H2O=NaAlO2+4H2��(��дΪNaH+H2O=NaOH+ H2��) D ���װ�õ������� ��������ɫ����ʱ ��ɫ�������� ���ܳ�ȥHCl�����ܽ���Cl2���ܽ�� ���� Al2O3 û��β������װ�ã�����ɴ�����Ⱦ
��������
(1)���⻯�ƺ��⻯����-1�۵�H������ˮ��Ӧ���ݴ˷��������ݷ�Ӧ���״̬����Ӧ����ѡ����ʵ�װ�ã�
(2)��Ũ������MnO2��ϼ�����ȡ��ȡCl2���Ƶõ�Cl2�к�������HCl��H2O����ͨ������ʳ��ˮ��ȥ�Ȼ������ʣ�Ȼ��ͨ��Ũ������и��Ȼ��ʹ�����������������ڼ���ʱ��Ӧ��ȡAlCl3���ݴ˷������
(1)���⻯�ƺ��⻯����-1�۵�H������ˮ��Ӧ����Ӧ����ʽΪ��NaAlH4+2H2O=NaAlO2+4H2��(��дΪNaH+H2O=NaOH+ H2��)��Ϊ��ֹ���ʣ���ȡ���⻯�Ʊ����ڷ�ˮ��Һ�н��У�AlC13���л��ܼ��γɵ���Һ��NaH�����������·�����ӦAlCl3+4NaH=NaAlH4+3NaCl��װ��A��B���ǹ������ʼ�����ȡ�����ʵ�״̬����Ӧ���������ϣ�C��D������״̬��������ȡNaAlH4������AlCl3�к��������ᾧˮ��ʪ��ˮ���ø��Ȼ�����ȡNaAlH4ʱ���ͻ�ͬʱ�����ܶȱȿ���С������������ֻ���������ſ����ķ����ռ���װ��C���õ��������ſ������������������������ռ���װ��D���ʣ��ʺ���ѡ����D��
(2)��������μӵĻ�ѧ��Ӧ��������װ�ú�Ӧ���еĵ�һ�������Ǽ��װ�õ������ԣ�Ϊ��֤����Ĵ�����Ӧ��D�г�������ɫ����ʱ�ټ��Ⱦƾ��ƣ��Ը���װ���п�������ֹAl��װ���ڿ����е�O2��Ӧ��
�ڱ���ʳ��ˮ�д����ܽ�ƽ�⣬������ˮ�д��ڻ�ѧƽ�⡣��HCl��������ˮ������ˮ��c(Cl-)����NaCl���ܽ�ƽ���Cl2��ˮ�����Ŀ��滯ѧ��Ӧ��ƽ����������ƶ����Ӷ��������������а�ɫ�����������ò���ͬʱ�ֽ������������ܽ�ȣ�
��Cװ��ʢ��Ũ���ᣬ�������Ǹ�������������Cװ�ã���ʪ��������Al�ڷ�Ӧʱ��������AlCl3��������ˮ��Ӧ�����ֱ�ΪAl(OH)3��HCl������ˮ�ֵ�������HCl�ӷ��������ΪAl(OH)3������Al(OH)3�����ֽⷴӦ����Al2O3�����Ԥ��ʵ���D���������A1C13����ܻ�����Al2O3��
(3)Cl2���ж����壬������������������������ɴ�����Ⱦ����˸�װ�ô��ڵ�����ȱ����û��β������װ�ã�����ɴ�����Ⱦ��

 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�����Ŀ��ijѧ����0.1000mol/L��NaOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
A��ȡ��NaOH��Һע���ʽ�ζ������̶���0����2cm��3cm��
B����ȡ25.00mL������������Һע��ྻ����ƿ��������2��3�η�̪��
C���ñ���Һ��ϴ�ζ���2��3�Σ�
D������Һ������0������0�����¿̶Ȳ����¶�����
E����ʢ�б���Һ�ļ�ʽ�ζ����ܶ��ã����ڵζ��ܼ���ʹ֮������Һ��
F������ƿ���ڵζ��ܵ����棬�ñ�NaOH��Һ�ζ����յ㲢�ǵζ���Һ��Ŀ̶ȡ�
�ݴ�ʵ������գ�
��1����ȷ���������˳����__��__��__��__��B��__�����������ĸ��д��
��2���ζ���ϴ��֮ǰ����___���ζ�������ʱ�������յζ��ܵĻ���������ҡ����ƿ���۾�ע��___���ζ��ﵽ�յ�ı�־��___��
��3������B�������֮ǰ�����ô�����Һ��ϴ��ƿ����Եζ������Ӱ����___������ƫ������ƫС����������������
��4�������Ĵεζ���ʼ�ͽ���ʱ���ζ����е�Һ����ͼ��ʾ��������NaOH��Һ�����Ϊ___mL��

��5��ijѧ�������Ĵ�ʵ���¼���ݼ����������Һ�����ʵ���Ũ�ȣ�c��HCl��=___��
�ζ����� | ���ᣨmL�� | 0.1000mol/LNaOH�������mL�� | ||
�ζ�ǰ | �ζ��� | ��Һ��� | ||
��һ�� | 25.00 | 0.05 | 26.17 | 26.12 |
�ڶ��� | 25.00 | 1.58 | 30.31 | 28.73 |
������ | 25.00 | 0.22 | 26.30 | 26.08 |