��Ŀ����
�����ֶ�����Ԫ�أ����ǵĽṹ�����ʵ���Ϣ���±�������
| Ԫ�� | �ṹ�����ʵ���Ϣ |
| A | �Ƕ�������(��ϡ��������)ԭ�Ӱ뾶����Ԫ�أ���Ԫ�ص�ij�ֺϽ���ԭ�ӷ�Ӧ�ѵĵ��ȼ� |
| B | B��Aͬ���ڣ�������������ˮ��������� |
| C | Ԫ�ص���̬�⻯�K������ˮ������������� |
| D | �Ǻ�ˮ�г��⡢��Ԫ���⺬������Ԫ�أ��䵥�ʻ���Ҳ������ˮ���������г��õ�����ɱ���� |
����ݱ�����Ϣ��д��
(1)Aԭ�ӵĺ�������Ų�ʽ ��
(2)BԪ�������ڱ��е�λ�� ��
���Ӱ뾶��B A(����ڡ���С�ڡ�)��
(3)Cԭ�ӵĵ����Ų�ͼ�� ����ԭ�Ӻ����� ��δ�ɶԵ��ӣ�������ߵĵ���Ϊ ����ϵĵ��ӣ������� �Ρ�
(4)Dԭ�ӵĵ����Ų�ʽΪ ��D���Ľṹʾ��ͼ�� ��
(5)B������������Ӧ��ˮ������A������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ ����D���⻯���ˮ���ﷴӦ�Ļ�ѧ����ʽΪ ��
(1)1s22s22p63s1
(2)��3���ڵڢ�A�� С��
(3) 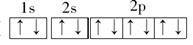 3 p �Ĵ�
3 p �Ĵ�
(4)1s22s22p63s23p5��[Ne]3s23p5
(5)NaOH��Al(OH)3=NaAlO2��2H2O 3HCl��Al(OH)3=AlCl3��3H2O
����

��ϰ��ϵ�д�
 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
�����Ŀ
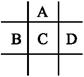
���в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
| Ԫ�ر�� | Ԫ�ص����ʻ�ԭ�ӽṹ |
| X | �����������Ǵ�����������3�� |
| Y | �����µ�����˫ԭ�ӷ��ӣ����⻯���ˮ��Һ�Լ��� |
| Z | ��������Ԫ�صļ������а뾶��С |
(1)Ԫ��X��һ�ֵ����������г�������ˮ�������������õ��ʵĻ�ѧʽ�� ��Ԫ��Z�����ӽṹʾ��ͼΪ ��
(2)Ԫ��Y����Ԫ���γ�һ������YH4+����д��������Һ�д��ڸ����ӵ�ʵ�����������ͽ��� ��
(3)д��ZԪ������������Ӧ��ˮ������NaOH��Һ��Ӧ�����ӷ���ʽ�� ��
(4)Ԫ��X��Ԫ��Y��ȣ��ǽ����Խ�ǿ���� (��Ԫ�ط��ű�ʾ)�����б�������֤����һ��ʵ���� ��
a��Y���⻯����ڴ�X2��ȼ������X���⻯���Y2
b��X�ĵ��ʱ�Y�ĵ��ʸ�������H2����
c��X��Y�γɵĻ�������YԪ�س�����̬
�±��г�ǰ20��Ԫ���е�ijЩԪ�����ʵ�һЩ���ݣ�
| Ԫ�� ���� | A | B | C | D | E | F | G | H | I | J |
| ԭ�Ӱ뾶 ��10��10m�� | 1.02 | 2.27 | 0.74 | 1.43 | 0.77 | 1.10 | 0.99 | 1.86 | 0.75 | 1.17 |
| ���̬ | ��6 | ��1 | �� | ��3 | ��4 | ��5 | ��7 | ��1 | ��5 | ��4 |
| ��ͼ�̬ | ��2 | �� | ��2 | �� | ��4 | ��3 | ��1 | �� | ��3 | ��4 |
�Իش��������⣺
��1������10��Ԫ���е�һ��������С����______�����ţ���
��2��д�������йط�Ӧ�Ļ�ѧ����ʽ��
��E�ĵ�����IԪ�ص�����������Ӧ��ˮ�����Ũ��Һ�ڼ��������µķ�Ӧ��____________________��
��H2C2��EC2��Ӧ��_____________________________________________ ��
��3������E��F��G����Ԫ���е�ij����Ԫ���γɵĻ������У�ÿһ��ԭ�Ӷ�����8�����ȶ��ṹ����________��д����ʽ������Ԫ��Bԭ��������5��Ԫ�ػ�̬ԭ�ӵ����Ų�ʽ��__________________��
��4��Ԫ��E��C����Ԫ�ؿ��γ�һ����Է�������Ϊ60��һԪ������ӡ��������EԪ��ԭ����____________��__________�ӻ��ɼ��������й��γ�____________���Ҽ���__________���м���
��5��C��I��Ƚϣ��ǽ����Խ�������________����Ԫ�����ƣ���������֤��Ľ��۵��������е�________�����ţ���
a����̬�⻯����ȶ��Ժͻӷ���
b�����ʷ����еļ���
c����Ԫ�صĵ縺��
d�������������
e���⻯����X��H���ļ�����X����C��I��Ԫ�أ�
f������������Ȼ��Ĵ���
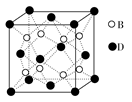
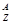 X��ʾԭ�ӣ�
X��ʾԭ�ӣ�