��Ŀ����
��I�����������ѳ�Ϊ��ǰ��δ����һ��ȫ���Կ��⡣Ϊ���Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���ܴ�ʹȼ��ѭ��ʹ�õĹ��룬��ͼ��ʾ��

��ش��������⣺
(1)���̢������ת����ʽΪ________��ת��Ϊ________�ܡ�
(2)����ת�������У���H1�ͦ�H2�Ĺ�ϵ��________��
(3)����1 mol��ѧ��������������±���
�����£�N2��H2O��Ӧ����NH3���Ȼ�ѧ����ʽΪ_______________________��
(II)��һ�Թ��м���0.01mol/L��KMnO4������Һ��0.1mol/LH2C2O4��Һ���ں����·������·�Ӧ��
2KMnO4+5 H2C2O4+3H2SO4��K2SO4+2MnSO4+10CO2+8H2O��5���Ӻ���Mn2+��Ũ��Ϊ0.004mol/L��
��4���Լ���0��5�����ڣ���(H2C2O4)��____________��
(5)�����Ӧ�ӿ�ʼ����һ��ʱ������ʡ�ʱ��ͼ����ͼ�� ���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________��
���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________��

��ش��������⣺
(1)���̢������ת����ʽΪ________��ת��Ϊ________�ܡ�
(2)����ת�������У���H1�ͦ�H2�Ĺ�ϵ��________��
(3)����1 mol��ѧ��������������±���
| ���ۼ� | H��N | H��O | N��N | O��O |
| ����1 mol��ѧ����������/(kJ��mol��1) | 393 | 460 | 941 | 499 |
(II)��һ�Թ��м���0.01mol/L��KMnO4������Һ��0.1mol/LH2C2O4��Һ���ں����·������·�Ӧ��
2KMnO4+5 H2C2O4+3H2SO4��K2SO4+2MnSO4+10CO2+8H2O��5���Ӻ���Mn2+��Ũ��Ϊ0.004mol/L��
��4���Լ���0��5�����ڣ���(H2C2O4)��____________��
(5)�����Ӧ�ӿ�ʼ����һ��ʱ������ʡ�ʱ��ͼ����ͼ��
 ���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________��
���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________����I����1��̫�� 1�֣���ѧ (1��)�� ��2����H1������H2 (1��)
��3��2N2(g)+6H2O(g)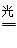 4NH3(g)+3O2(g) ��H��+1189kJ/mol
4NH3(g)+3O2(g) ��H��+1189kJ/mol (4��)
(4��)
����4��0.002md/(L��min) (2��)
��5��t1��t2˵��Mn2+��������t2��t3����Ӧ��Ũ�Ƚ��� (4��)
��3��2N2(g)+6H2O(g)
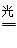 4NH3(g)+3O2(g) ��H��+1189kJ/mol
4NH3(g)+3O2(g) ��H��+1189kJ/mol (4��)
(4��)����4��0.002md/(L��min) (2��)
��5��t1��t2˵��Mn2+��������t2��t3����Ӧ��Ũ�Ƚ��� (4��)
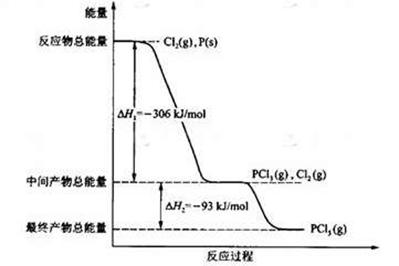
�����������I����1������ת��ʾ��ͼ��֪������I������̫���ܽ�������ˮ��CO2��ת��Ϊ�������������������״��ͼ���ȣ��������ת����̫����ת��Ϊ��ѧ�ܡ�
��2�����̢�����һ���������������������������״��ͼ����������ת��ΪCO2��ˮ�͵����ȣ����Ը��������غ��֪��H1������H2��
��3������ԭ���غ��֪��������ˮ��Ӧ���ɰ�����ͬʱ�����������ɣ���Ӧ�Ļ�ѧ����ʽΪ2N2+6H2O
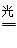 4NH3(g)+3O2�����ڷ�Ӧ�ȵ��ڶϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����÷�Ӧ�ķ�Ӧ�ȡ�H��2��942kJ/mol��6��2��460kJ/mol��4��3��393kJ/mol��3��499kJ/mol����1189kJ/mol�����Ȼ�ѧ����ʽΪ2N2(g)+6H2O(g)
4NH3(g)+3O2�����ڷ�Ӧ�ȵ��ڶϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����÷�Ӧ�ķ�Ӧ�ȡ�H��2��942kJ/mol��6��2��460kJ/mol��4��3��393kJ/mol��3��499kJ/mol����1189kJ/mol�����Ȼ�ѧ����ʽΪ2N2(g)+6H2O(g)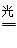 4NH3(g)+3O2(g) ��H��+1189kJ/mol��
4NH3(g)+3O2(g) ��H��+1189kJ/mol������4��5���Ӻ���Mn2+��Ũ��Ϊ0.004mol/L ������ݷ���ʽ��֪���IJ�������ʵ���Ũ����0.004mol/L�� ��0.01mol/L�����Է�Ӧ���ʦ�(H2C2O4)��0.01mol/L ��5min��0.002md/(L��min)��
��5������Ӱ�췴Ӧ���ʵ�����һ�����¶ȡ�Ũ�Ⱥʹ����ȣ���ϵ�¶Ȳ��䣬����Ӱ�췴Ӧ���ʵ����ؿ��Դ�Ũ�Ⱥʹ����ĽǶȷ�����t1��t2��Ӧ�������ߣ�˵��Mn2+���������ӿ��˷�Ӧ���ʣ���t2��t3��Ӧ�����ֽ��ͣ���˵����Ӧ��Ũ�Ƚ��͵��·�Ӧ���ʽ��͡�

��ϰ��ϵ�д�
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ
 R(g) ��H = ��Q1 kJ��mol��1����2R (g)+N(g)
R(g) ��H = ��Q1 kJ��mol��1����2R (g)+N(g) kJ
kJ


 ������������±���ʾ��
������������±���ʾ��

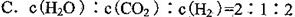

 1���� ������Ӧ�¶���T1���ߵ�T2��ƽ��ʱPCl5�ķֽ���Ϊ
1���� ������Ӧ�¶���T1���ߵ�T2��ƽ��ʱPCl5�ķֽ���Ϊ

 2SO3 ���ɷֵ����ʵ����仯��t2ʱ�̸ı���������������������������
2SO3 ���ɷֵ����ʵ����仯��t2ʱ�̸ı���������������������������
 �ı仯���
�ı仯���