��Ŀ����
��24�֣���ͼ��Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���д���пո�
������10��Ԫ���У�ԭ�Ӱ뾶��С����________(��Ԫ�ط���)����ѧ��������õ�Ԫ����_____(�Ԫ�ط��š�)������һ�ֺ��ؿɲⶨ������������ֺ��صķ����� ��
��Ԫ�آٵ�ԭ�ӽṹʾ��ͼΪ__________��Ԫ�آٵ����������ṹʽΪ��________��Ԫ�آ�ĵ��ʵ���ʽΪ��__________��
���õ���ʽ��ʾ������ܺ͢���γɹ���
 ________________________________________________��
________________________________________________��
��Ԫ�آݵ������������ᷴӦ�����ӷ���ʽΪ�� _________________________________��
Ԫ�آݵĵ���������������Һ��Ӧ�Ļ�ѧ����ʽΪ�� ________________________________��
��Ԫ�آݵĵ�����Fe��ϡ���ṹ��ԭ��أ���������ķ����ڻ���ԭ���װ��ͼ�����ԭ��صĵ缫���Ϻ͵������Һ����д�������ĵ缫��ӦΪ___________________________��

��5�� Ԫ�آ��������ˮ��ˮҺ�� ɫ��������ͨ��Ԫ�آߵ�ij���������Һ��ɫ��ȥ���û�ѧ����ʽ��ʾԭ�� ��
Ԫ�آ��������ˮ��ˮҺ�� ɫ��������ͨ��Ԫ�آߵ�ij���������Һ��ɫ��ȥ���û�ѧ����ʽ��ʾԭ�� ��
��6��Ԫ�آߵ�������ۺ�����۷ֱ�Ϊ �� �� ������һ�������£�Ԫ�آ���H2��Ӧ��һ����(������Ϊ��Ӧ���еij̶�)�����ж�����ͬ������Ԫ�آ���H2��Ӧ����(ѡ���������С������ͬ��) ��������
| | | | |||||||||||||||
| | | | | �� | �� | �� | �� | | |||||||||
| | �� | �� | | | �� | �� | �� | ||||||||||
| | | | | | | | | | | | | | | | �� | | |
| | | | | | | | | | | | | | | | | | |
��Ԫ�آٵ�ԭ�ӽṹʾ��ͼΪ__________��Ԫ�آٵ����������ṹʽΪ��________��Ԫ�آ�ĵ��ʵ���ʽΪ��__________��
���õ���ʽ��ʾ������ܺ͢���γɹ���
 ________________________________________________��
________________________________________________����Ԫ�آݵ������������ᷴӦ�����ӷ���ʽΪ�� _________________________________��
Ԫ�آݵĵ���������������Һ��Ӧ�Ļ�ѧ����ʽΪ�� ________________________________��
��Ԫ�آݵĵ�����Fe��ϡ���ṹ��ԭ��أ���������ķ����ڻ���ԭ���װ��ͼ�����ԭ��صĵ缫���Ϻ͵������Һ����д�������ĵ缫��ӦΪ___________________________��

��5��
 Ԫ�آ��������ˮ��ˮҺ�� ɫ��������ͨ��Ԫ�آߵ�ij���������Һ��ɫ��ȥ���û�ѧ����ʽ��ʾԭ�� ��
Ԫ�آ��������ˮ��ˮҺ�� ɫ��������ͨ��Ԫ�آߵ�ij���������Һ��ɫ��ȥ���û�ѧ����ʽ��ʾԭ�� ����6��Ԫ�آߵ�������ۺ�����۷ֱ�Ϊ �� �� ������һ�������£�Ԫ�آ���H2��Ӧ��һ����(������Ϊ��Ӧ���еij̶�)�����ж�����ͬ������Ԫ�آ���H2��Ӧ����(ѡ���������С������ͬ��) ��������
��24�֣�
(1) F, Ar ��14C ��3�֣��� (2)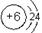 ��O="C=O" ��
��O="C=O" ��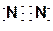 ��3�֣���
��3�֣���
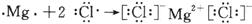 ��2�֣���
��2�֣���
(3) Al2O3 + 6H+="2" Al3++3H2O��2Al+2NaOH+2H2O=2NaAlO2+3H2����4�֣�
(4)
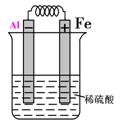
Al��3e����Al3����2�֣���2�֣���
(5) dz����ɫ��SO2 + Cl2 + 2 H2O = H2SO4 + 2 H Cl ��2�֣�
(6) +6��1�֣��� -2 ��1�֣�����С��1�֣�
(1) F, Ar ��14C ��3�֣��� (2)
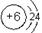 ��O="C=O" ��
��O="C=O" ��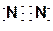 ��3�֣���
��3�֣���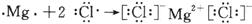 ��2�֣���
��2�֣���(3) Al2O3 + 6H+="2" Al3++3H2O��2Al+2NaOH+2H2O=2NaAlO2+3H2����4�֣�
(4)

Al��3e����Al3����2�֣���2�֣���
(5) dz����ɫ��SO2 + Cl2 + 2 H2O = H2SO4 + 2 H Cl ��2�֣�
(6) +6��1�֣��� -2 ��1�֣�����С��1�֣�
��

��ϰ��ϵ�д�
�����Ŀ

 ��֮�ͣ�Cԭ��������������Dԭ��������������4����
��֮�ͣ�Cԭ��������������Dԭ��������������4���� 19
19 ʵ�飬����������ļ�ơ���֪������BGO��,�ദ�������̬������BGO��,��ļ�̬��������γ�ij�ֹ����Ȼ���ʱ���ʵļ�̬��ͬ���ڴ��Ȼ���������������8�������ȶ��ṹ����BGO�ɿ����������������Ԫ�ص����������γɵĸ������������BGO����Ļ�ѧʽ��,����������������������������ͬ��
ʵ�飬����������ļ�ơ���֪������BGO��,�ദ�������̬������BGO��,��ļ�̬��������γ�ij�ֹ����Ȼ���ʱ���ʵļ�̬��ͬ���ڴ��Ȼ���������������8�������ȶ��ṹ����BGO�ɿ����������������Ԫ�ص����������γɵĸ������������BGO����Ļ�ѧʽ��,����������������������������ͬ��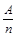 ��KΪA��n�ı�ֵ��������������ȷ����
��KΪA��n�ı�ֵ��������������ȷ����