��Ŀ����
�����±��ṩ��ʵ�������������(�Dz���������ѡ)���ܹ��ﵽ��Ӧʵ��Ŀ�ĵ���(����)
| ѡ�� | ʵ������������� | ʵ��Ŀ�� |
| A | ��ʽ�ζ��ܡ���ʽ�ζ��ܡ��ձ�����ƿ | ��0.01 mol/L��ϡ����궨δ֪NaOH��Һ��Ũ�� |
| B | ��Һ©������ƿ������ܡ����ܡ�����ƿ | �ü�ʯ�Һ�Ũ��ˮ�Ʊ�����NH3 |
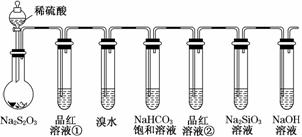
| C | ��Ũ�����̼��ϼ��ȣ�ֱ�ӽ����ɵ�����ͨ��������ʯ��ˮ�У�ʯ��ˮ����� | �������������CO2�Ĵ��� |
| D | ��ij��Һ�м���BaCl2��Һ��������ɫ���� | ����SO |
������ѡAB��C����SO2�и��ţ�D����Ag���и��š�

 ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д� ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д��������������������γ��������Ҫ���ʡ�ij�������п��ܺ����������ӣ�Na����Ba2����NH ��Al3����Cl����SO
��Al3����Cl����SO ��SO
��SO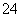 ��NO
��NO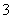 �ȡ�ij�о�С��ȡ�õ�һ���������꣬Ũ�������ó�����Һ�ֳ����ݣ���������ʵ�飺
�ȡ�ij�о�С��ȡ�õ�һ���������꣬Ũ�������ó�����Һ�ֳ����ݣ���������ʵ�飺
| ���� | �����Լ� | ʵ������ |
| ��һ����Һ | �μ������ĵ���KI��Һ | ��Һ����ɫ |
| �ڶ�����Һ | �μ��������ữ��BaCl2��Һ | �а�ɫ�������� |
| ��������Һ | �μ�NaOH��Һ�����ȣ������NaOH��Һ���(V)�����ɵij�������������������ʵ���(n)�Ĺ�ϵ����ͼ |
|
��ش��������⣺
(1)����ʵ�����жϸ������п϶������ڵ�������______________������ȷ����������________________��
(2)д����һ����Һ�μӵ���KI��Һʱ������Ӧ�����ӷ���ʽ��__________________��
(3)��������Һ�μ�NaOH��Һ�����ȣ����������з����˶����Ӧ��д������������Ӧ�����ӷ���ʽ��__________________________________��__________________________��
(4)���ʵ�鷽����������������Ƿ����Cl����___________________________________
______________________________��
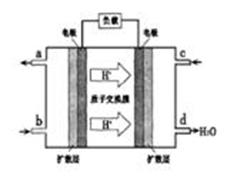
(5)��С��Ϊ��̽��NO����������������γɹ��̣�����ƿ�г��뺬������NO��SO2���壬������ͨ��O2��������ѧ��Ӧ����������������ˮ�������������꣬��NO���� ����Ӧ�е�������________________________________________________________��
����Ӧ�е�������________________________________________________________��
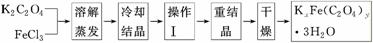
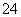 ת��ΪH2C2O4����0.100 0 mol��L��1KMnO4��Һ�ζ���������KMnO4��Һ24.00 mLʱǡ����ȫ��Ӧ��H2C2O4������KMnO4��Һ��Ӧ�Ļ�ѧ����ʽ�ǣ�__________________________��������Һ�м��������Ļ�ԭ����ǡ�ý�Fe3����ȫת��ΪFe2������KMnO4��Һ�����ζ�����Fe2����ȫ����ʱ����ȥKMnO4��Һ4.00 mL���˵ζ���Ӧ�����ӷ���ʽ��____________��
ת��ΪH2C2O4����0.100 0 mol��L��1KMnO4��Һ�ζ���������KMnO4��Һ24.00 mLʱǡ����ȫ��Ӧ��H2C2O4������KMnO4��Һ��Ӧ�Ļ�ѧ����ʽ�ǣ�__________________________��������Һ�м��������Ļ�ԭ����ǡ�ý�Fe3����ȫת��ΪFe2������KMnO4��Һ�����ζ�����Fe2����ȫ����ʱ����ȥKMnO4��Һ4.00 mL���˵ζ���Ӧ�����ӷ���ʽ��____________��