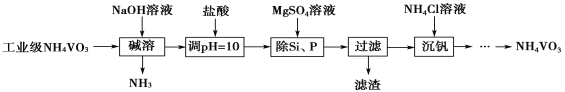
��Ŀ����
����Ŀ��T ��ʱ�����ݻ�Ϊ2 L��3�������ܱ������з�����Ӧ��3A(g)��B(g)![]() nC(g)������ͬ��ʽͶ�뷴Ӧ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
nC(g)������ͬ��ʽͶ�뷴Ӧ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
���� | �� | �� | �� |
��Ӧ���Ͷ���� | 3 mol A��2 mol B | 6 mol A��4 mol B | 2 mol C |
�ﵽƽ���ʱ��/min | 5 | 8 | |
A��Ũ��/mol��L��1 | c1 | c2 | |
C���������% | w1 | w3 | |
���������ܶ�/g��L��1 | ��1 | ��2 |
����˵����ȷ����(����)
A. ��n<4����2c1<c2
B. ��n��4����w3��w1
C. ����n��ֵ�Ƕ��پ���2��1����2
D. �����״ﵽƽ�������ʱ��������Ҵﵽƽ�������ʱ���
���𰸡�C
��������
A. ���º��������ҵ�ЧΪ�ڼ���ƽ�������ѹǿ����һ������n<4��������ӦΪ���������С�ķ�Ӧ��ƽ�������ƶ������з�Ӧ��ת���ʸ���A�з�Ӧ��ת���ʣ�����2c1>c2����A����
B. ��n=4����Ӧǰ�������������䣬��ʼֻͶ��C����ʼͶ�ϱ�n(A):n(B)=3:1Ϊ��Чƽ�⣬ƽ��ʱͬ�����ʵİٷֺ�����ȣ���������ʼͶ�ϱ�Ϊ3:2�����Լͱ����ǵ�Чƽ�⣬��w3��w1����B����
C. ��ʼʱ����ͬ�����ʵ�Ͷ�����Ǽ�2�������������غ㶨�ɿ�֪��������������Ϊ����2�����������ݻ��������һ������2��1����2����C��ȷ��
D. ���з�Ӧ�����ʼŨ�ȴ�Ӧ���ʸ��죬����ƽ��ʱ����̣����������״ﵽƽ�������ʱ��������Ҵﵽƽ�������ʱ�䳤����D����ѡC��

����Ŀ��ijС���о�AgCl���ܽ�ƽ�⣺��10 mL 1mol/L KCl ��Һ�м���1 mL 0.2 mol/LAgNO3��Һ��������Һ����Ϊ2�ݣ���������ʵ�飺
ʵ����� | ʵ����� | ʵ������ |
I | ������һ�ݻ���Һ���ˣ�����Һ�з���Mg��
| i. ��Һ���������ü��������ж�������� ii. ����Mg������������ɫ���ݲ����������ȼ����Һ�г��ְ�ɫ���ǡ� iii. һ��ʱ���ʼ�����غ�ɫ�����Mg������Ҳ��Ϊ�غ�ɫ���������ݵ����ʱ仺���� |
II | ����һ�ݻ���Һ�з����С��ͬ��Mg��
| iv. �غ�ɫ������������Զ���ʵ��I��Mg�������غ�ɫ�������������ii��iii��ͬ�� |
��֪��AgOH���ȶ��������ֽ�ΪAg2O���غ�ɫ���غ�ɫ����Ag��Ϊ��ɫ��AgCl��Ag2O������Ũ��ˮ����Ag(NH3)2��
��1����Һ������ɢϵΪ________��
��2������ii����ɫ���ݲ�����ԭ����_________��д����ѧ����ʽ����
��3������iii�У����²������ݵ������½�����ҪӰ��������________��
��4������ΪMg��������غ�ɫ������һ����Ag��Ag2O����������Ag�����ӷ���ʽΪ____��
��5�������ʵ�����Ag��ȡʵ��I�б����Ϊ�غ�ɫ��Mg�����Թ��У������м��������Լ�a����Ӧ�������������м���Ũ���ᣬ������ɫ���壬��Һ���а�ɫ�����
�� ��ɫ������Ϊ_____���ѧʽ������ɫ���������ԭ����_____��д���ӷ���ʽ����
�� �Լ�aΪ________�������Լ�a��Ŀ����________��
�� ��ʵ����֤���غ�ɫ������һ����Ag��ʵ��������________��
��6����һ�����ʵ����֤��Mg��������غ�ɫ��������Ag2O��ʵ�鷽���ǣ�ȡʵ��I�б����Ϊ�غ�ɫ��Mg��_________��
��7���ۺ�����ʵ�飬��˵������AgCl(s) ![]() Ag+(aq) + Cl��(aq)��֤�ݼ�������________��
Ag+(aq) + Cl��(aq)��֤�ݼ�������________��
����Ŀ��̼�͵��Ļ����������������й㷺���ڡ��ش��������⣺
(1)��ת������ʹ����β���е���Ҫ��Ⱦ�CO��NOx��̼�⻯����������Ӧ�����������ʣ���������β����Ⱦ��
��֪��N2(g)+O2(g)=2NO(g) ��H1=+180.5 kJ/mol��
2C(s)+O2(g)=2CO(g) ��H2=��221.0 kJ/mol��
C(s)+O2(g)=CO2(g) ��H3=��393.5 kJ/mol
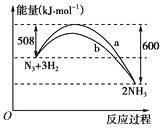
��β��ת����Ӧ2NO(g) +2CO(g)=N2(g)+2CO2(g)����H=___________��
(2)����һ����Ҫ�Ļ���ԭ�ϣ��ڹ�ũҵ�������й㷺��Ӧ�á���773 Kʱ���ֱ�2.00 mol N2��6.00 mol H2����һ���̶��ݻ�Ϊ1 L���ܱ������У����ŷ�Ӧ�Ľ��У�����������n(H2)��n(NH3)�뷴Ӧʱ��(t)�Ĺ�ϵ�����ʾ��
t/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
n(H2)/mol | 6.00 | 4.50 | 3.60 | 3.30 | 3.03 | 3.00 | 3.00 |
n(NH3)/mol | 0 | 1.00 | 1.60 | 1.80 | 1.98 | 2.00 | 2.00 |
�ٸ��¶��£�����ͬ�ݻ�����һ������Ͷ��N2��H2��NH3����Ũ�Ⱦ�Ϊ3 mol/L�����ʱv��____v��(������������С��������������)��
���ɱ��е�ʵ�����ݿɵõ���c��t���Ĺ�ϵ����ͼ��ʾ����ʾc(N2)��t��������______���ڴ��¶��£�����ʼ����4 mol N2��12 mol H2����Ӧ�մﵽƽ��ʱ����ʾc(H2)��t����������Ӧ�ĵ�Ϊ________��

(3)NOx���ŷ���Ҫ����������β�����������÷�ӦC(s)+2NO(g)![]() N2(g)+CO2(g) ��H=-34.0kJ/mol���û���̿��NO������������֪���ܱ������м���������C��һ������NO���壬���ֺ�ѹ���NO��ת�������¶ȵı仯��ͼ��ʾ��
N2(g)+CO2(g) ��H=-34.0kJ/mol���û���̿��NO������������֪���ܱ������м���������C��һ������NO���壬���ֺ�ѹ���NO��ת�������¶ȵı仯��ͼ��ʾ��
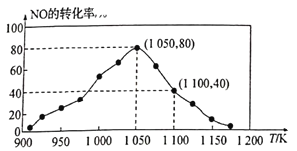
����ͼ��֪����1100Kʱ��CO2���������Ϊ__________��
����ij���ʵ�ƽ���ѹ���������ʵ���Ũ��Ҳ���Ա�ʾ��ѧƽ�ⳣ��(����Kp)����1050K��1.1��106Paʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Kp=______[��֪�������ѹ(P��)=������ѹ(Pa)���������]��
(4)�����Ե������Һ�У��Զ��Բ������缫����CO2ת��Ϊ��ϩ![]() ԭ����ͼ��ʾ
ԭ����ͼ��ʾ
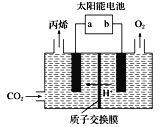
��̫���ܵ�صĸ�����_______(����a������b��)
�����ɱ�ϩ�ĵ缫��Ӧʽ��___________��