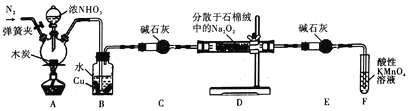
题目内容
【题目】氯磺酰氰酯(结构简式为 )是一种多用途的有机合成试剂,在HClO4-NaClO4介质中, K5[Co3+O4W12O36](简写为Co3+W)可催化合成氯磺酰氰酯。
)是一种多用途的有机合成试剂,在HClO4-NaClO4介质中, K5[Co3+O4W12O36](简写为Co3+W)可催化合成氯磺酰氰酯。
(1)基态钴原子的核外电子排布式为________________。组成HClO4-NaClO4的4种元素的电负性由小到大的顺序为____________________。
(2)氯磺酰氰酯分子中硫原子和碳原子的杂化轨道类型分别是____________、___________, 1个氯磺酰氰酯分子中含有σ键的数目为______________,氯磺酰氰酯中5种元素的第一电离能由大到小的顺序为_________________。
(3)ClO4-的空间构型为__________________。
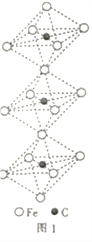
(4)一种由铁、碳形成的间隙化合物的晶体结构如图1所示,其中碳原子位于铁原子形成的八面体的中心,每个铁原子又为两个八面体共用,则该化合物的化学式为________________。
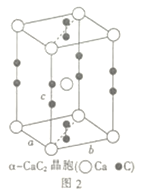
(5)电石(CaC2)是制备氯化氰(ClCN)的重要原料。四方相碳化钙(CaC2)的晶胞结构如上图2所示,其晶胞参数分别为a、b、c,且a=b,c=640pm。已知四方相碳化钙的密度为1.85g·cm-3,[C≡C]2-中键长为120pm,则成键的碳原子与钙原子的距离为________pm和_______pm。(设阿伏加德罗常数的数值为6×1023)
【答案】 1s22s22p63s23p63d7{或[Ar]3d7} Na<H<Cl<O sp3 sp 5 N>O>Cl>C>S 正四面体 Fe3C 260 306
【解析】(1)1s22s22p63s23p63d7{或[Ar]3d7};Na<H<Cl<O
(2)从氯磺酰氰酯的结构简式可以看出来,S原子形成了4个σ键,没有孤电子对,所以S原子采取了sp3杂化,C原子形成了一个单键和一个三键,共有两个σ键,没有孤电子对,所以C原子是sp杂化。1个氯磺酰氰酯分子中含有σ键的数目为5个,其中包含的五种元素的第一电离能大小顺序为:N>O>Cl>C>S,故答案为:sp3;sp;5;N>O>Cl>C>S。
(3)ClO4-中心原子Cl最外层共有8个电子,与4个O形成了4个σ键,VSEPR模型为正四面体型,没有孤电子对,所以ClO4-的空间构型为正四面体型。
(4)每个八面体中包含了一个C原子,一共6个Fe每个Fe被两个八面体公用,所以化学式为:Fe3C。
(5)侧面碳原子与钙原子的距离![]() ,一个晶胞中包含了Ca:1+8×1/8=2,C2:2×1/2+4×1/4=2,一个晶胞中包含了2个CaC2,所以一个晶胞的质量为:
,一个晶胞中包含了Ca:1+8×1/8=2,C2:2×1/2+4×1/4=2,一个晶胞中包含了2个CaC2,所以一个晶胞的质量为:![]() ,又因为
,又因为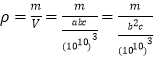 可以求出
可以求出![]() ;底面碳原子与钙原子的距离
;底面碳原子与钙原子的距离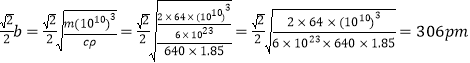
故答案为:260;306。

 导学教程高中新课标系列答案
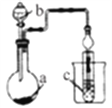
导学教程高中新课标系列答案【题目】如图装置,其中试剂b为浓硫酸,则下列实验设计能达到实验目的的是
选项 | 试剂a | 试剂c | 实验目的 |
|
A | MnO2和NaCl | 紫色石蕊溶液 | 验证Cl2的漂白性 | |
B | 蔗糖 | 澄清石灰水 | 验证CO2的生成 | |
C | FeS | 溴水 | 验证非金属性:Br>S | |
D | Na2SO3 | 品红溶液 | 验证SO2的漂白性 |
A. A B. B C. C D. D