��Ŀ����
����Ŀ������ѧ����ѡ��3�����ʽṹ�����ʡ��绯ѧ��ԭ������һ�ִ���CO2����ɫ�������з�չDZ���ķ�����ͭ��������(In)�Ƚ������Ǹ÷�Ӧ�Ĵ�����
(1)InԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽΪ____________����CuԪ��ͬ���ڣ��һ�̬ԭ����2��δ�ɶԵ��ӵĹ���Ԫ����_________________(��Ԫ�ط���)��
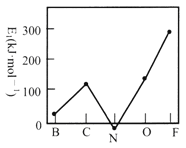
(2)��һ��������(E1)��Ԫ�صĻ�̬��̬ԭ�ӵõ�һ�������γ���̬��һ������ʱ���ų����������ڶ����ڲ���Ԫ�ص�E1�仯������ͼ��ʾ���Է���̼Ԫ�ص�E1�ϴ��ԭ��_____________________________��
(3)[PtC14(NH3)2]��Nԭ�ӵ��ӻ���ʽΪ________��������ѧ�����Ͱ���_______(����ĸ)��
a�����Ӽ�b�����ۼ�c��������d����λ��e�����
[PtCl4(NH3)2]��H-N-H��֮��н�______(�>����<����=��)NH3������H-N-H��֮��нǣ�ԭ����__________________________________��
(4)C60����ɲ�ȡ�����ܶѻ���Ȼ���ڿ�϶�в���������ӻ�ó����壬��ͼΪK3C60������������������K+ռ�ݵ���C60Χ�ɵ����������϶��_____��϶����C60���ӵ�ԭ����������ֱ�ΪA(0��0��0)��B(![]() )��C(1��1��1)�ȣ������Aλ��C60���������K+��ԭ���������Ϊ_____________���жϾ����۷е�ߵͣ�C60_________K3C60(�>����<����=��)��
)��C(1��1��1)�ȣ������Aλ��C60���������K+��ԭ���������Ϊ_____________���жϾ����۷е�ߵͣ�C60_________K3C60(�>����<����=��)��

���𰸡� 5s25p1 TiNi ̼ԭ�ӽ��һ�����Ӻ��2p3���Ϊ�����״̬���ȶ��Խ�ǿ sp3 bd > [PtC14(NH3)2]�γɹ����У�NH3�е�Nԭ�ӵŶԵ�����Pt4+�γ�����λ����ת��Ϊ�ɼ����Ӷԣ��������ɼ����ӶԵij������ͣ����Լ������� �������� ![]() <
<
��������������������⿼��۵����Ų�ʽ����д����һ����������ԭ���ӻ���ʽ���жϡ���ѧ�����жϡ������ķ����������۵�ߵ͵ıȽϡ�
��1��Inλ��Ԫ�����ڱ��е�������IIIA�壬��̬Inԭ�ӵļ۵����Ų�ʽΪ5s25p1��Cu���ڵ������ڣ���Cuͬ�����һ�̬ԭ����2��δ�ɶԵ��ӵĹ���Ԫ����Ti��Ni��
��2����̬̼ԭ�ӵļ۵����Ų�ʽΪ2s22p2��̼ԭ�ӵõ�һ�����Ӻ��2p3���Ϊ�����״̬���ȶ��Խ�ǿ���ų��������ϴ�����̼Ԫ�ص�E1�ϴ���
��3��[PtCl4��NH3��2]��ÿ��Nԭ���γ�3������Ҽ�����Pt4+�γ�1����λ������λ��Ҳ���ڦҼ���Nԭ����û�йµ��Ӷԣ�Nԭ�ӵ��ӻ���ʽΪsp3�ӻ���[PtCl4��NH3��2]��N��H֮���γɹ��ۼ���Pt4+��Cl-��NH3���Ӽ��γ���λ����[PtCl4��NH3��2]������ѧ�����ͣ����ۼ�����λ������ѡbd��[PtCl4��NH3��2]��NH3������Nԭ�Ӷ�����sp3�ӻ���[PtCl4��NH3��2]�γɹ����У�NH3�е�Nԭ���ϵŵ��Ӷ���Pt4+�γ�����λ����ת��Ϊ�ɼ����Ӷԣ��������ɼ����ӶԵij������ͣ����Լ���������[PtCl4��NH3��2]��H-N-H��֮��н�![]() NH3������H-N-H��֮��нǡ�
NH3������H-N-H��֮��нǡ�
��4������ͼʾC60λ�ھ�����8�������6�����ģ��á���̯������1�������к�C60��8![]() +6
+6![]() =4�����ݻ�ѧʽK3C60��1�������к�12��K+������8��K+ռ��C60Χ�ɵ����������϶��4��K+ռ��C60Χ�ɵ����������϶���������϶�����ķֱ������ĺ������ϣ�������Aλ��C60���������K+λ��ͼ
=4�����ݻ�ѧʽK3C60��1�������к�12��K+������8��K+ռ��C60Χ�ɵ����������϶��4��K+ռ��C60Χ�ɵ����������϶���������϶�����ķֱ������ĺ������ϣ�������Aλ��C60���������K+λ��ͼ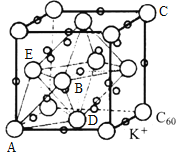 ��ABDEΧ�ɵ����������϶����C60���ӵ�ԭ����������ֱ�ΪA��0��0��0����B��
��ABDEΧ�ɵ����������϶����C60���ӵ�ԭ����������ֱ�ΪA��0��0��0����B��![]() ��0��
��0��![]() ����C��1��1��1���ȣ���ԭ���������D��
����C��1��1��1���ȣ���ԭ���������D��![]() ��
��![]() ��0����E��0��
��0����E��0��![]() ��
��![]() ��������Aλ��C60���������K+��ԭ���������Ϊ��
��������Aλ��C60���������K+��ԭ���������Ϊ��![]() ��
��![]() ��
��![]() ����C60���ڷ��Ӿ��壬K3C60�������Ӿ��壬���Ӽ�������С�����Ӽ��������۷е�ߵͣ�C60
����C60���ڷ��Ӿ��壬K3C60�������Ӿ��壬���Ӽ�������С�����Ӽ��������۷е�ߵͣ�C60![]() K3C60��
K3C60��

 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�