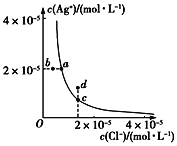
题目内容
【题目】某工业废催化剂含有SiO2、ZnO、CuS、ZnS、Fe3O4等物质,为落实“节约资源,变废为宝”的环保理念,某课外兴趣小组的同学取20g该物质进行实验,回收其中的Cu和Zn,实验方案如下:
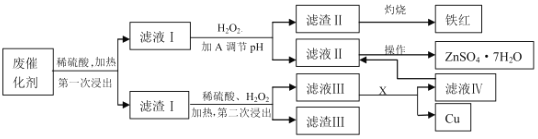
已知:ZnS可与稀硫酸反应;CuS不溶于稀硫酸,也不与其发生反应。请回答下列问题:
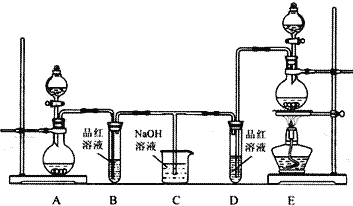
(1)可用图装置进行第一次浸出,烧杯中盛放的是______溶液。
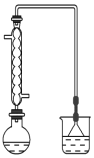
(2)滤液Ⅰ中的Fe2+最好用______来检验。
a.氯水 b.双氧水 c.KSCN溶液 d.K3[Fe(CN)6]溶液
(3)物质A是含有X元素的氧化物(XO),则X是______(填元素符号),由滤液Ⅱ、Ⅳ滤液获得ZnSO47H2O的操作是______________。
(4)第二次浸出时的化学方程式为_______________。
(5)加A调节溶液的pH约为______时,可以完全除去其中的杂质离子。
(当溶液中离子浓度小于等于10-5mol/L时视为沉淀完全;实验条件下部分物质的溶度积常数为:Ksp[Fe(OH)3]=10-38,Ksp[Fe(OH)2]=10-17,Ksp[Zn(OH)2]=10-17,Ksp[Cu(OH)2]=10-20)
(6)实验最后获得了5.74gZnSO47H2O晶体(假设实验中没有损耗),但不能由此确定原催化剂中锌元素的质量分数,原因是_________________。
【答案】NaOH溶液或者氨水 d Zn 蒸发浓缩,冷却结晶(过滤,洗涤,干燥) CuS+H2O2+H2SO4=CuSO4+S+2H2O 3 在实验过程中添加了单质锌,不能由产品质量来计算锌元素的质量分数
【解析】
废催化剂含有SiO2、ZnO、CuS、ZnS、Fe3O4等物质,取20g该物质进行实验,加入稀硫酸并加热进行浸取,可溶解ZnO、ZnS、Fe3O4等物质,滤液Ⅰ含有ZnSO4、FeSO4、Fe2(SO4)3,加入过氧化氢可氧化亚铁离子生成铁离子,调节pH生成滤渣Ⅱ为Fe(OH)3,灼烧生成Fe2O3,滤液Ⅱ含有ZnSO4,经蒸发浓缩结晶得到粗ZnSO47H2O,滤渣Ⅰ含有SiO2、CuS,向盛有滤渣1的反应器中加H2SO4和H2O2溶液,发生氧化还原反应,生成硫酸铜、硫,滤渣Ⅲ含有硫和二氧化硅,滤液Ⅲ含有硫酸铜,加入锌置换,可生成硫酸锌和铜,以此解答该题。
(1)第一次浸出可生成硫化氢等气体,可用NaOH溶液或者氨水吸收,防止污染环境;
(2)亚铁离子可与K3[Fe(CN)6]溶液反应生成蓝色沉淀,因此可用K3[Fe(CN)6]溶液检验Fe2+的存在,故合理选项是d;
(3)X为Zn,由滤液Ⅱ、Ⅳ滤液获得ZnSO47H2O,可进行蒸发浓缩,冷却结晶(过滤,洗涤,干燥)等操作;
(4)第二次浸出可氧化CuS生成硫、硫酸铜,化学方程式为CuS+H2O2+H2SO4=CuSO4+S+2H2O,故答案为:CuS+H2O2+H2SO4=CuSO4+S+2H2O;
(5)加入ZnO调节pH除去铁离子,应生成氢氧化铁沉淀,Ksp[Fe(OH)3]=10-38,可知c(OH-)=![]() mol/L=10-11mol/L,则c(H+)=
mol/L=10-11mol/L,则c(H+)=![]() 10-3mol/L,所以溶液的pH=3;
10-3mol/L,所以溶液的pH=3;
(6)因在实验过程中添加了单质锌,则不能由产品质量来计算锌元素的质量分数。