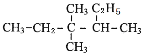��Ŀ����
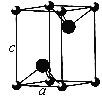
����Ŀ����֪NixO���徧���ṹΪNaCl�ͣ���ͼ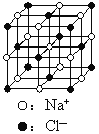 �������ھ���ȱ�ݣ�xֵ��С��1����֪NixO�����ܶ���Ϊ5. 71g��cm��3�������߳�Ϊ4.28��10��10m��Ni�����ԭ������Ϊ58.7��O�����ԭ������Ϊ16��������֪��4.283=78.4 ��
�������ھ���ȱ�ݣ�xֵ��С��1����֪NixO�����ܶ���Ϊ5. 71g��cm��3�������߳�Ϊ4.28��10��10m��Ni�����ԭ������Ϊ58.7��O�����ԭ������Ϊ16��������֪��4.283=78.4 ��![]() =1.414��
=1.414��
��1������������Ni����֮�����̾���___________����ȷ��0.01����
��2����O2����������ҵȾ����Ni����Χ�ɵļ�������״_______��
��3����NixO��xֵΪ_________����ȷ��0.01����
��4�������е�Ni�ֱ�ΪNi2����Ni3������˾���Ļ�ѧʽ_________��
���𰸡�3.03��10-10m �������� 0.88 Ni0.64Ni0.24O
��������
��1������������Ni����֮�����̾���Ϊ��Խ��ߵ�![]() ��
��
��2����O2-��������ҵȾ����Ni����Χ�����������͡�
��3���ɾ����߳����㾧�����������ܶȼ��㾧������������һ������NixO��Ħ��������
��4������ԭ���غ�͵���غ���ʽ���㡣
��1������������Ni����֮�����̾���Ϊ��Խ��ߵ�![]() ������������Ni����֮�����̾���Ϊ
������������Ni����֮�����̾���Ϊ![]() ��4.28��10-10m=3.03��10-10m��
��4.28��10-10m=3.03��10-10m��
��2����O2-��������ҵȾ����Ni������6�����ֱ�λ��O2-�����¡����ҡ�ǰ������Χ�����������͡�
��3�����������Ϊ��4.28��10-10m��3=7.84��10-29m3������������Ϊ5.71g/cm3��7.84��10-23cm3=4.47664��10-22g��������̯����1�������к�4��O2-����M��NixO��=![]() ��6.02��1023mol-1=67.37g/mol����58.7x+16=67.37�����x=0.88��
��6.02��1023mol-1=67.37g/mol����58.7x+16=67.37�����x=0.88��
��4������Ni�غ㣬n��Ni2+��+n��Ni3+��=0.88�����ݵ���غ㣬2n��Ni2+��+3n��Ni3+��=2�����n��Ni2+��=0.64��n��Ni3+��=0.24���˾���Ļ�ѧʽΪNi0.64Ni0.24O��