��Ŀ����
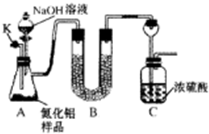 �����մɵ���������Al2O3���»�ԭ���Ʊ���Al2O3+3C+N
�����մɵ���������Al2O3���»�ԭ���Ʊ���Al2O3+3C+N
| ||
��1����ԭ�ϽǶȷ�����AlN�е����ʿ�����
��2��װ��B�е��Լ���
��3����ַ�Ӧ�����Kͨ��һ��ʱ��N2����Ŀ����
��4��������Ը�ʵ��ĸ����ʩ�У�������߲ⶨ���ȷ�ȵ���
a���ڼ���NaOH��Һ֮ǰ���ž�װ���ڵ�����
b���μ�NaOH��Һ���˹���
c��ȡ��Bװ��
d����C֮������ʢ�м�ʯ�ҵĸ����
��5��ʵ����ȷ��ȡm g����Ʒ���ݣ��������βⶨ�����ʵ��ǰ��װ��Cƽ������n g������Ʒ��AlN�Ĵ���Ϊ
��6��ʵ��֤��������ͼװ�òⶨ��AlN����ƫ�ߣ�ԭ����
��������1����ԭ���õ��Ĺ���������������������Ŀ��Ϣ��AlN����ǿ����Һʱ������NH3����д����ʽ��
��2�������ĸ����ü�ʯ�ң�����©�����Է�ֹ������
��3����������������ʵ������ȷ�Ĺؼ����������Խ�����ȫ���ų��Ա��������գ���
��4����������������ȷ�ⶨ��ʵ������ȷ�Ĺؼ�������ʵ��Ŀ����ѡ��
��5�����ݵ�ԭ�ӵ��غ㣬���������ʵ�������AlN�����ʵ����������AlN������������
��6���ⶨ��AlN����ƫ�ߣ���������ƫ�ߣ���������������������ɣ�
��2�������ĸ����ü�ʯ�ң�����©�����Է�ֹ������
��3����������������ʵ������ȷ�Ĺؼ����������Խ�����ȫ���ų��Ա��������գ���
��4����������������ȷ�ⶨ��ʵ������ȷ�Ĺؼ�������ʵ��Ŀ����ѡ��
��5�����ݵ�ԭ�ӵ��غ㣬���������ʵ�������AlN�����ʵ����������AlN������������
��6���ⶨ��AlN����ƫ�ߣ���������ƫ�ߣ���������������������ɣ�
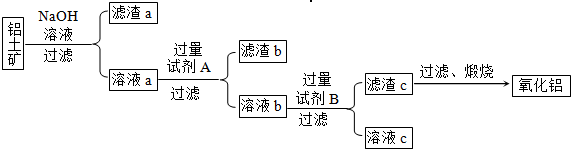
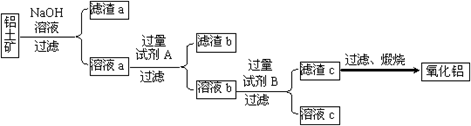
����⣺��1����ԭ���õ��Ĺ�����������������̼�����Լ������������Ե������е�����ֻ������������̼��������Ŀ��Ϣ��AlN����ǿ����Һʱ������NH3����ѧ����ʽΪ��AlN+NaOH+H2O=NaAlO2+NH3�����ʴ�Ϊ����������̼��AlN+NaOH+H2O=NaAlO2+NH3����
��2�������ĸ����ü�ʯ�ң���װ���У�����©�����Է�ֹ�������ʴ�Ϊ����ʯ�ң���ֹ������
��3����������������ʵ������ȷ�Ĺؼ���Ϊ��֤���ɵİ���ȫ�����ų������������գ��ʴ�Ϊ���������Խ�����ȫ���ų��Ա��������գ�
��4��a��װ���ڵ��������������������Ӱ�죬�����ڼ���NaOH��Һ֮ǰ���ž�װ���ڵ�������������ȷ���أ���a��ȷ��
b���μ�NaOH��Һ���˹��죬���Ա�֤�������ܺ��������Ƴ�ַ�Ӧ�ų�����������������ʵ���ȷ���йأ���b����
c��ȡ��Bװ�ã���Ũ�������յ��ǰ����Լ�ˮ��������ʹ�ý��ƫ�ߣ�Ӱ����ȷ�ȣ���c��ȷ��
d����C֮������ʢ�м�ʯ�ҵĸ���ܣ����Է�ֹ�����е�ˮ�������ţ����ȷ�ȣ���d����
��ѡac��
��5��ʵ��ǰ��װ��Cƽ������ng�������ɰ�����������ng����ԭ�ӵ��غ㣬���������ʵ�������AlN�����ʵ�����AlN����������Ϊ��
=
���ʴ�Ϊ��
��
��6���ⶨ��AlN����ƫ�ߣ������������ֵƫ�ߣ�������װ��C�е�Ũ���������˿����е�ˮ�������£��ʴ�Ϊ��װ��C�е�Ũ���������˿����е�ˮ������
��2�������ĸ����ü�ʯ�ң���װ���У�����©�����Է�ֹ�������ʴ�Ϊ����ʯ�ң���ֹ������
��3����������������ʵ������ȷ�Ĺؼ���Ϊ��֤���ɵİ���ȫ�����ų������������գ��ʴ�Ϊ���������Խ�����ȫ���ų��Ա��������գ�
��4��a��װ���ڵ��������������������Ӱ�죬�����ڼ���NaOH��Һ֮ǰ���ž�װ���ڵ�������������ȷ���أ���a��ȷ��
b���μ�NaOH��Һ���˹��죬���Ա�֤�������ܺ��������Ƴ�ַ�Ӧ�ų�����������������ʵ���ȷ���йأ���b����
c��ȡ��Bװ�ã���Ũ�������յ��ǰ����Լ�ˮ��������ʹ�ý��ƫ�ߣ�Ӱ����ȷ�ȣ���c��ȷ��
d����C֮������ʢ�м�ʯ�ҵĸ���ܣ����Է�ֹ�����е�ˮ�������ţ����ȷ�ȣ���d����
��ѡac��
��5��ʵ��ǰ��װ��Cƽ������ng�������ɰ�����������ng����ԭ�ӵ��غ㣬���������ʵ�������AlN�����ʵ�����AlN����������Ϊ��
| ||
| m |
| 41n |
| 17m |
| 41n |
| 17m |
��6���ⶨ��AlN����ƫ�ߣ������������ֵƫ�ߣ�������װ��C�е�Ũ���������˿����е�ˮ�������£��ʴ�Ϊ��װ��C�е�Ũ���������˿����е�ˮ������
������������һ���������ʵ���ɺͺ����ⶨ֪ʶ���ۺϿ����⣬Ҫ��ѧ�����з����ͽ��������������Ѷȴ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ����AlN +��CO����ƽ��
����AlN +��CO����ƽ��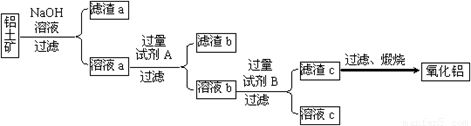
 ����AlN +��CO����ƽ��
����AlN +��CO����ƽ��