��Ŀ����
��18�֣����������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2�����ʡ�
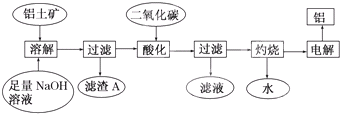
������������ȡ����������������ͼ��ʾ��
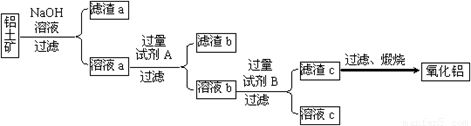
��1���Լ�A���������������� ����Һb���Լ�B��Ӧ�����ӷ���ʽΪ�������������� ��
��2������NaOH��Һ���еķ�Ӧ�����ӷ���ʽΪ������������������ ___��
������������������������������������������������Һa��ͨ�������CO2�����õ��IJ��������պ�Ҳ�ɵõ�Al2O3���÷�����ȱ�������������������������� ��
������ڵ����������Ʊ�������
��3��д�����Ļ�ѧ����ʽ���������������������������������� ��ÿ����0.27������������ת�Ƶ��ӵ����ʵ���Ϊ���������������� mol��
�������մɵ����������������ַ����Ʊ�
��4�������������»�ԭ������Al2O3 +��C +�� N2  ����AlN +��CO����ƽ��
����AlN +��CO����ƽ��
���Ȼ����백�����ºϳɷ���AlCl3+NH3  AlN+3HCl
AlN+3HCl
��5�������ڱȷ������������ϸ������ơ�����˵���У���ȷ���������������� ��
A���������е� Al2O3��C��N2�ṹ�ȶ�����Ӧʱ�ƻ���ѧ����Ҫ���ĸ��������
B���������е�Al2O3��C���ײ����ڵ�������
C�����ַ����е�������Ϊ��ԭ����
��18�֣�
��(1)���ᣨ��������ᣩ�� Al3++3NH3��H2O =Al(OH)3��+3NH4+
��2��Al2O3��2OH����2AlO2����H2O SiO2��2OH����SiO32����H2O
Al2O3���SiO2����
��3��2Al2O3�����ڣ� 4Al + 3O2��
3��104
4Al + 3O2��
3��104
��4�� 1 Al2O3 + 3 C + 1 N2 === 2 AlN + 3 CO ��5�� AB
�����������������Һ��Ӧʱ�������������������ܽ⣬��������������a����Ҫ�ɷ֣���Һ��������ǿ�ᣨ��������ᣩ������������ܣ��˳�Ϊ����b����Һb����Ҫ�ɷ�Ϊƫ��������Һ������Һb��ͨ�������CO2���壬������������������c������Һc�ijɷ�Ϊ̼�����ƣ�������c������ˮ�ɵ���������
��2������Һa��ͨ�������CO2���γɵ������н����������ʣ����ἰ�������������Ⱥ�õ���Al2O3�к���SiO2����
��3��2Al2O3�����ڣ� 4Al + 3O2�� ����12������ת�ơ�
4Al + 3O2�� ����12������ת�ơ�
��4������Ԫ�صĻ��ϼ������غ����ƽ��C��0����+2�ۣ���N��0������3�ۡ�
��5�����ݸ���Ӧ�P������������ʼ����ϼ۵����������ж���

| A�����������Ҫ�ɷ���Fe2O3 | B�����������Ҫ�ɷ���Al2O3 | C����ҵ�Ʊ�������Ҫ���õ�ⱥ��ʳ��ˮ�ķ��� | D��������ʯұ�������Ĺ����У���ԭ����Ҫ�ǽ�̿ |
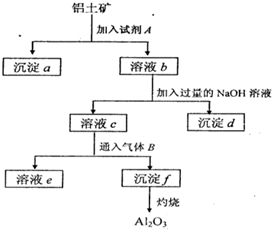
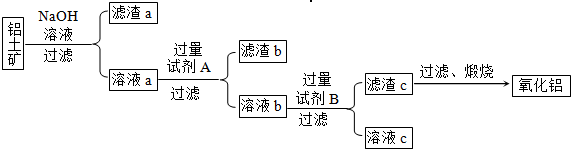
 ����һ����Ҫ�Ľ������������������о���������Ҫ����;����ͼ�Ǵ����������Ʊ����Ĺ������̣�
����һ����Ҫ�Ľ������������������о���������Ҫ����;����ͼ�Ǵ����������Ʊ����Ĺ������̣�