��Ŀ����
14��HAλ��ͬ��һԪ�ᣬ��HA��Һ��NaOH��Һ������ʵ�飮��1����c��HA��=c��NaOH��=0.1mol/L������Һ�������ϣ���û�Ϻ���Һ��pH��7����HAΪ���ᣨ�ǿ�ᡱ�����ᡱ������ʾ��ҺpH��7��ԭ������ӷ���ʽ��A-+H2O?HA+OH-��
��2����HAΪǿ�ᣬc��NaOH��=0.1mol/L������Һ�������ϣ������Һ��pH=12������ǰc��HA��=0.08mol/L��
��3����c��HA��=0.04mol/L��c��NaOH��=0.02mol/L������Һ�������ϣ������Һ��pH��7���������йػ����Һ��˵������ȷ����ABC��
A��HA����NaA��ˮ�⣬NaA����HA�ĵ��� B��c��Na+����c��A-��
C��c��HA��+c��A-��=0.02mol/L D��c��A-����c��HA��
��4����HAΪHCl���ñ�NaOH��Һ�ζ�ʱ�������������������c��HCl��ƫ�����B��
A��������ˮϴ����ƿ��δ���T�������Һ���еζ�
B����ʽ�ζ�����ˮϴ�Ӻ�ֱ��ʢ�ű�NaOH��Һ�ζ�
C���ζ�ǰ���Ӷ������ζ����Ӷ�����
���� ��1��ǿ��������ˮ���Լ��ԣ�
��2������Һ�������ϣ������Һ��pH=12�����Ϻ���Һ�е�����������Ũ��Ϊc��OH-��=$\frac{c��NaOH����V-c��HA����V}{V+V}$=10-2mol/L���ݴ˼��㣻
��3��c��HA��=0.04mol/L��c��NaOH��=0.02mol/L������Һ�������ϣ���Һ�е�����Ϊ�����ʵ�����NaA��HA�������Һ��pH��7��˵���ε�ˮ��̶ȴ��ڴ�����ĵ���̶ȣ���ϵ���غ�������غ������
��4������c��HCl��=$\frac{c��NaOH����V��NaOH��}{V��HCl��}$������
��� �⣺��1����c��HA��=c��NaOH��=0.1mol/L������Һ�������ϣ�����ǡ�÷�Ӧ�����Σ���û�Ϻ���Һ��pH��7��˵������Ϊǿ�������Σ�ˮ���Լ��ԣ���HAΪ���ᣬ���ε�ˮ�����ӷ���ʽΪ��A-+H2O?HA+OH-��
�ʴ�Ϊ�����A-+H2O?HA+OH-��
��2����HAΪǿ�ᣬc��NaOH��=0.1mol/L������Һ�������ϣ������Һ��pH=12�����Ϻ���Һ�У�c��OH-��=10-2mol/L����c��OH-��=$\frac{c��NaOH����V-c��HA����V}{V+V}$=10-2mol/L�����c��HA��=0.08mol/L��
�ʴ�Ϊ��0.08��
��3��c��HA��=0.04mol/L��c��NaOH��=0.02mol/L������Һ�������ϣ���Һ�е�����Ϊ�����ʵ�����NaA��HA�������Һ��pH��7��˵���ε�ˮ��̶ȴ��ڴ�����ĵ���̶ȣ�
A��HA����NaA��ˮ�⣬NaA����HA�ĵ��룬��������ƣ���A��ȷ��
B����Һ�еĵ���غ�Ϊc��Na+��+c��H+��=c��A-��+c��OH-������֪ pH��7����c��H+����c��OH-��������c��Na+����c��A-������B��ȷ��
C������Һ�������ϣ�HA��NaA����Ũ�ȱ�Ϊԭ����һ�룬��c��HA��+c��A-��=0.02mol/L����C��ȷ��
D���ε�ˮ��̶ȴ��ڴ�����ĵ���̶ȣ���c��A-����c��HA������D����
�ʴ�Ϊ��ABC��
��4����HAΪHCl���ñ�NaOH��Һ�ζ�ʱ��c��HCl��=$\frac{c��NaOH����V��NaOH��}{V��HCl��}$��
A��������ˮϴ����ƿ��δ���T�������Һ���еζ���������Һ�����ʵ������䣬���ĵ��������Ʋ��䣬���Բ�ý�����䣬��A��ѡ��
B����ʽ�ζ�����ˮϴ�Ӻ�ֱ��ʢ�ű�NaOH��Һ�ζ��������������Ƶ�Ũ��ƫС���ζ�ʱ���ĵ��������Ƶ����ƫ����ⶨ���ƫ��B��ȷ��
C���ζ�ǰ���Ӷ������ζ����Ӷ������������������Ƶ����ƫС���ɼ��㹫ʽ��֪������õ��������Ũ��ƫС����C����
�ʴ�Ϊ��B��
���� ���⿼�����ε�ˮ�⡢������ʵĵ��롢��Һ���غ��ϵ��Ӧ�á�����к͵ζ����������ȣ���Ŀ�ۺ��Խ�ǿ���Ѷ��еȣ�ע�ضԳ���������ۺ�Ӧ�õĿ��飮

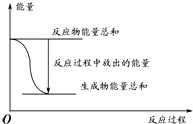
| A�� | ��Ӧ������������ϵ����ͼ��ʾ����÷�ӦΪ���ȷ�Ӧ | |
| B�� | �����÷�Ӧ��Ƴ�ԭ��أ�пΪ���� | |
| C�� | ��ѧ��Ӧ���ʱ��뷴Ӧ����ʽ�ļ������й� | |
| D�� | ���������Ϊԭ��أ�����32.5gп�ܽ�ʱ�������ų�����һ��Ϊ11.2 L |
| A�� | ���Ȼ�����·����ķ�Ӧ�����ȷ�Ӧ | |
| B�� | ���ȷ�Ӧ���Է��ģ����ȷ�Ӧ�����Է��� | |
| C�� | �����Ƶ�ˮ���H��0������ĵ����H��0 | |
| D�� | ��ѧ��Ӧ�е������仯Դ�ڻ�ѧ���Ķ��Ѻ��γ� |
| ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1molN2��3molH2 | 2molNH3 | 4molNH3 |
| NH3��Ũ�ȣ�mol•L-1�� | c1 | c2 | c3 |
| ��Ӧ�������仯 | �ų�akJ | ����bkJ | ����ckJ |
| ��ϵѹǿ��Pa�� | p1 | p2 | p3 |
| ��Ӧ��ת���� | ��1 | ��2 | ��3 |
��1����1+��2=1��
��2��a+b=92.4��
��3��2c1��c3
��4��2p2��p3
��5����1+��3=��1��
| A�� | ����ǿ����H4SiO4��H3PO4��H2SO4 | B�� | ��ԭ�ԣ�Na��Mg��Al | ||
| C�� | ���ȶ��ԣ�SiH4��PH3��H2S��HCl | D�� | �۵㣺SiO2��NaCl��I2��CO2 |
| Ԫ�ر�� | Ԫ��������ԭ�ӣ�����ӣ��ṹ |
| T | M���K����1������ |
| X | ��������Ԫ�صĽ��������а뾶��С |
| Y | �����������Ǵ�����������3�� |
| Z | Z���⻯������������������ˮ���ﷴӦ����һ���� |
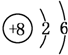 ��
����2��Ԫ��T��Ԫ��X��ȣ������Խ�ǿ����Na����Ԫ�ط��ű�ʾ����д��T��X����Ԫ�ص�����������ˮ�������Ӧ�����ӷ���ʽ��OH-+Al��OH��3=AlO2-+2H2O��
��3��Ԫ��Y ���⻯��е��ͬ������Ԫ�ص��⻯��ߣ�ԭ���ǣ�ˮ���Ӽ���������
��4��T��X��Y��Z������Ԫ�����γɼ������Ӽ����зǼ��Թ��ۼ��Ļ����д���û�����ĵ���ʽ
 ��
�� | A�� |  ���Ȼ�狀�����������NH 3 | B�� | 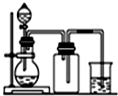 ��ͭƬ��ϡ������NO | ||
| C�� | 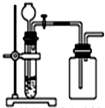 ��п����ϡ������H 2 | D�� | 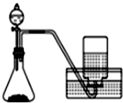 ��˫��ˮ�Ͷ���������O2 |