��Ŀ����
����Ŀ��ijʵ�����Ժ�������(��Ҫ�ɷ�ΪNiO������������ FeO��Fe2O3��CoO��BaO ��SiO2)Ϊԭ���Ʊ�NixOy ��̼����(CoCO3)�Ĺ���������ͼ��
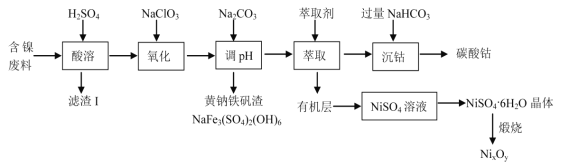
��֪�й��������↑ʼ�����ͳ�����ȫ�� pH �����
�������� | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Ni(OH)2 |
��ʼ������ pH | 1.5 | 6.5 | 7.2 | 7.7 |
������ȫ�� pH | 3.7 | 9.7 | 9.4 | 9.2 |
��ش��������⣺
(1)������������Ҫ�ɷ�Ϊ(�ѧʽ)_____��
(2)��������ʱ(���� NaClO3)�����ӷ���ʽΪ_____��
(3)���� Na2CO3 ������ pH����Ŀ�������ɻ���������������ȥ����Ҳ��ͨ������MO(��Ӧ�Ľ���������)������ pH ������ȥ���� pH ���Ƶķ�ΧΪ_____��
(4)��ʵ������ȡ����Ӧ����_____�����(����������)����ˮ���м���NaHCO3 ʱ�����ӷ���ʽΪ_________��
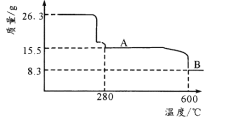
(5)�� NiSO4 ��Һ���NiSO4��6H2O ����IJ��������ǣ�_____��_________�����ˣ�ϴ�ӣ������������ʱʣ������������¶ȱ仯������ͼ���������� B ������ʾ�����������Ϊ_____��
���𰸡�BaSO4��SiO2 ![]() 3.7��pH��7.2 ��Һ©��
3.7��pH��7.2 ��Һ©�� ![]() ����Ũ�� ��ȴ�ᾧ ����������
����Ũ�� ��ȴ�ᾧ ����������
��������
ԭ�ϵijɷֺ���NiO��FeO��Fe2O3��CoO��BaO��SiO2������Ҫ�Ʊ����ֲ��һ�������������һ����CoCO3������FeO��![]() ��BaO��SiO2Ҫ���Ʊ���������ȥ������һ�������ܽ�ԭ�ϣ����ǵ������ķ�Ӧ���Ϳɵ�֪��������Һ�ijɷ֡�����л��ṩ���й��������↑ʼ�����ͳ�����ȫ��pH�ı�����������֪��Ҫ��ȥ����Ԫ�أ�Ӧ������Ԫ��ȫ��ת��Ϊ�������ٵ���pH�����ڲ����Ͳ�Ʒ������ͬʱ����������Ԫ�ء�������ʧ�������ж����ʵ���ɣ���ͨ��ԭ���غ���м��㣬�ٽ�һ���жϼ��ɡ�
��BaO��SiO2Ҫ���Ʊ���������ȥ������һ�������ܽ�ԭ�ϣ����ǵ������ķ�Ӧ���Ϳɵ�֪��������Һ�ijɷ֡�����л��ṩ���й��������↑ʼ�����ͳ�����ȫ��pH�ı�����������֪��Ҫ��ȥ����Ԫ�أ�Ӧ������Ԫ��ȫ��ת��Ϊ�������ٵ���pH�����ڲ����Ͳ�Ʒ������ͬʱ����������Ԫ�ء�������ʧ�������ж����ʵ���ɣ���ͨ��ԭ���غ���м��㣬�ٽ�һ���жϼ��ɡ�
(1)ԭ�ϵijɷ���NiO��FeO��Fe2O3��CoO��BaO��SiO2�������������ܣ�SiO2����Ӧ�����Ի�����������У�BaO�����ձ��BaSO4����������Ҳ������������У�
(2)���������Ŀ���ǽ�������ת����������������ں��������г�������ȥ���������ӷ���ʽΪ![]() ��
��
(3)����Na2CO3��һ����Ϊ�˳�ȥ����ͬʱҲҪ��������Ԫ�ص���ģ�����ѡ���pH����Ϊ3.7~7.2��
(4)��ȡ��ؼ��������Ƿ�Һ©��������̼�����ƺ���֮һ��CoCO3���������ǵ�NaHCO3�ǹ����ģ��������յ����ӷ���ʽΪ![]() ��
��
(5)����Һ��ȡ�ᾧ�ij�������ǣ�����Ũ������ȴ�ᾧ������ϴ�Ӹ�������������Ħ��������263g/mol������26.3g��Ϊ0.1mol��280�濪ʼ��������������٣����ʱ������Ϊ�ᾧˮ��ʧ���µģ�����A�ζ�Ӧ�����ʾ���0.1mol��NiSO4���¶ȵ�600�棬����������ٴ��½����õ����յ��������������ԭ���غ㣬8.3g��������Ni������Ϊ5.9g����ôO������Ϊ2.4g��0.15mol�����Թ�����������![]() �����Թ��������T������������
�����Թ��������T������������

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д�