��Ŀ����
17��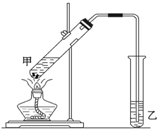 ��ʵ���������Ҵ����������Ũ�������Ʊ�һ������ζ�����ʣ�ʵ��װ����ͼ��ʾ�������Ҫ������
��ʵ���������Ҵ����������Ũ�������Ʊ�һ������ζ�����ʣ�ʵ��װ����ͼ��ʾ�������Ҫ��������1��д���÷�Ӧ�Ļ�ѧ����ʽ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$ CH3COOCH2CH3+H2O��Ӧ���ͣ�������Ӧ����ȡ����Ӧ����
��2�������Թ��м������Ƭ�ɷ�ֹ���У�������ʢ���Լ�Ϊ����̼������Һ������Һ����Ҫ�����ǣ����ջӷ�������������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�������
��3��ʵ������������������Ҵ���ȡijһ����Һ���Թܣ��μ�����ʯ��ָʾ��������Һ��죬����ȡ����ҺΪ���ᣬ����һ��Ϊ�Ҵ���
��4����װ��ͼ����һ�����ԵĴ����ǵ����ܵij������뵽Һ�����£�
���� ��1���Ҵ���������Ũ���������������¼��ȷ��ڷ�Ӧ��������������ˮ����Ӧ����ȡ����Ӧ��
��2��Ϊ��ֹҺ����ȱ�����Ҫ�����ʯ�����Ƭ�����ɵ����������ڱ���̼������Һ�г��ӷֲ㣻
��3�����������Һ�����ԣ��Ҵ��Ƿǵ���ʣ�
��4�������ܵij������뵽����̼������ҺҺ�����£�����������
��� �⣺��1���Ҵ���������Ũ���������������¼��ȷ��ڷ�Ӧ��������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH $?_{��}^{Ũ����}$ CH3COOCH2CH3+H2O����Ӧ����ȡ����Ӧ��
�ʴ�Ϊ��CH3COOH+CH3CH2OH $?_{��}^{Ũ����}$ CH3COOCH2CH3+H2O��������Ӧ����ȡ����Ӧ����
��2��Ϊ��ֹҺ����ȱ�����Ҫ�����ʯ�����Ƭ�����ɵ����������ڱ���̼������Һ�г��ӷֲ㣬������ʢ���Լ�Ϊ����̼������Һ�����������ջӷ�������������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�������
�ʴ�Ϊ�����Ƭ������̼������Һ�����ջӷ�������������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�������
��3�����������Һ�����ԣ��Ҵ��Ƿǵ���ʣ�ȡijһ����Һ���Թܣ��μ�����ʯ��ָʾ��������Һ��죬����ȡ����ҺΪ���ᣬ����һ��Ϊ�Ҵ���
�ʴ�Ϊ��ȡijһ����Һ���Թܣ��μ�����ʯ��ָʾ��������Һ��죬����ȡ����ҺΪ���ᣬ����һ��Ϊ�Ҵ���
��4����װ��ͼ����һ�����ԵĴ����ǵ����ܵij������뵽����̼������ҺҺ�����£�����������
�ʴ�Ϊ�������ܵij������뵽Һ�����£�
���� ���⿼����������Ӧԭ�����Ʊ����ɷ�����פ���dz����Լ��������жϣ�װ�õ���ȷ���������ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���NaOH��Һ�������Ƶ�Cu��OH��2����Һ���۵�ˮ��
| A�� | �� | B�� | �ڢ� | C�� | �٢ڢ� | D�� | �٢� |
��O2 ��Cl2 ��F2 ��H2 ��NaOH��Һ ��H2O��
| A�� | �٢ڢ� | B�� | �٢ڢۢ� | C�� | �ۢ� | D�� | ������ |
| A�� | ���Ļ�ѧ�����ȶ� | B�� | �������������²���Ӧ | ||
| C�� | ��������Ԫ�� | D�� | �����������γ�һ�����ܵ�����Ĥ |
| ѡ�� | ���������� | ��Һ |
| A | ����ͨ�뷴ӦC+2H2O+2Cl2$\frac{\underline{\;\;��\;\;}}{\;}$CO2+4HCl���ɵ����壬����ǣ�����ͨ�룬������ʧ | Ca��OH��2��Һ |
| B | ͨ��SO2��SO3������壬��Һ����ǣ����κ��������� | Ba��NO3��2��Һ |
| C | ͨ��SO2����Һ����ǣ�����ͨSO2��������������ʧ���ټ�������NaOH��Һ���ֱ���� | Ca��OH��2��Һ |
| D | ͨ�루NH4��2CO3���ȷֽ���������壬����ǣ�����ͨ�룬���Dz���ʧ | Ca��OH��2��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 2NO2��g��?N2O4 ��g�� | |
| B�� | 2NO2��g��?2NO ��g��+O2��g�� | |
| C�� | 2HI��g��?H2 ��g��+I2��g�� | |
| D�� | FeCl3��aq��+3KSCN��aq��?Fe��SCN��3��aq��+3KCl��aq�� |
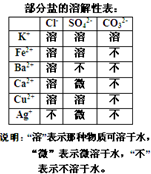 ij������������ˮ��Һ�У����ܺ������������������֣�K+��Ca2+��Fe2+��Cu2+��Cl-��CO32-��SO42-����ÿ��ȡ����Һ100.00mL��Һ��������ʵ�飺
ij������������ˮ��Һ�У����ܺ������������������֣�K+��Ca2+��Fe2+��Cu2+��Cl-��CO32-��SO42-����ÿ��ȡ����Һ100.00mL��Һ��������ʵ�飺