��Ŀ����
10������ָ����Ӧ�����ӷ���ʽ��ȷ���ǣ�������| A�� | FeCl3��Һ������ˮ����Fe��OH��3���壺Fe3++3OH-�TFe��OH��3�����壩 | |
| B�� | ICl�������ϡKOH��Һ�У�ICl+2OH-�TCl-+IO-+H2O | |
| C�� | �ö��Ե缫���CuSO4��Һ��2Cu2++4OH-$\frac{\underline{\;���\;}}{\;}$2Cu��+O2��+2H2O | |
| D�� | Ca��HCO3��2��Һ�����NaOH��Һ��Ӧ��HC${{O}_{3}}^{-}$+Ca2++OH-�TCaCO3��+H2O |
���� A��ˮ�ⷴӦΪ���淴Ӧ��
B��������������ԭ��Ӧ������NaCl��NaIO��
C����Ӧ����Cu�����������
D����Ӧ����̼���ơ�̼��ƺ�ˮ��
��� �⣺A��FeCl3��Һ������ˮ����Fe��OH��3��������ӷ�ӦΪFe3++3H2O?Fe��OH��3�����壩+3H+����A����
B��ICl�������ϡKOH��Һ�е����ӷ�ӦΪICl+2OH-�TCl-+IO-+H2O����B��ȷ��
C���ö��Ե缫���CuSO4��Һ�����ӷ�ӦΪ2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu��+O2��+4H+����C����
D��Ca��HCO3��2��Һ�����NaOH��Һ��Ӧ�����ӷ�ӦΪ2HCO3-+Ca2++2OH-�TCaCO3��+2H2O+CO32-����D����
��ѡB��
���� ���⿼�����ӷ�Ӧ����ʽ��д�������жϣ�Ϊ��Ƶ���㣬���շ����ķ�Ӧ�����ӷ�Ӧ����д����Ϊ���Ĺؼ�������������ԭ��Ӧ��ˮ�����ⷴӦ�����ӷ�Ӧ���飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
�����Ŀ
1��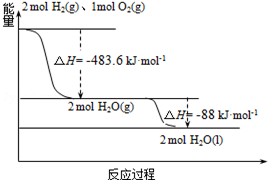 ����˹�á�����-M�������ػ���ɹ��������ߡ�����������Ԥ������������õ����ػ��ʹ�õ�����Һ��Ϊȼ�ռ���Һ��Ϊ�������ĸ��ܵ����ƽ�������֪��
����˹�á�����-M�������ػ���ɹ��������ߡ�����������Ԥ������������õ����ػ��ʹ�õ�����Һ��Ϊȼ�ռ���Һ��Ϊ�������ĸ��ܵ����ƽ�������֪��
��1��H2��g��=H2��l����H1=-0.92kJ•mol-1
��2��O2��g��=O2��l����H2=-6.84kJ•mol-1
��3����ͼ������˵����ȷ���ǣ�������
 ����˹�á�����-M�������ػ���ɹ��������ߡ�����������Ԥ������������õ����ػ��ʹ�õ�����Һ��Ϊȼ�ռ���Һ��Ϊ�������ĸ��ܵ����ƽ�������֪��
����˹�á�����-M�������ػ���ɹ��������ߡ�����������Ԥ������������õ����ػ��ʹ�õ�����Һ��Ϊȼ�ռ���Һ��Ϊ�������ĸ��ܵ����ƽ�������֪����1��H2��g��=H2��l����H1=-0.92kJ•mol-1
��2��O2��g��=O2��l����H2=-6.84kJ•mol-1
��3����ͼ������˵����ȷ���ǣ�������
| A�� | 2mol H2��g����1mol O2��g���������������2molH2O��g��������������� | |
| B�� | ������ȼ����Ϊ��H=-241.8 kJ•mol-1 | |
| C�� | �����Һ��ȼ�յ��Ȼ�ѧ����ʽΪ��2H2��l��+O2��l���T2H2O��g����H=-474.92kJ•mol-1 | |
| D�� | H2O��g�����H2O��l���Ĺ����У��ϼ����յ�����С�ڳɼ��ų������� |
18���йػ�ѧ������ȷ���ǣ�������
| A�� | Beԭ�ӵĽṹʾ��ͼ��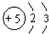 | |
| B�� | ��������ķ���ʽ��SiO2 | |
| C�� | ������Ϊ53��������Ϊ78�ĵ�ԭ�ӿɱ�ʾΪ��53131I | |
| D�� | ��ϩ�Ľṹʽ��CH2=CH2 |
5�����й��ڻ�ѧ����ı�ʾ��ȷ���ǣ�������
| A�� | ˮ�������ӵĵ���ʽ��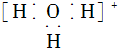 | B�� | �����ӵĽṹʾ��ͼ�� | ||
| C�� | ������Ϊ28�ĸ�ԭ�ӣ�2028Ca | D�� | �۱�ϩ�Ľṹ��ʽ��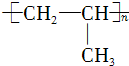 |
15��25��ʱ�������й���Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A�� | 0.1 mol•L-1CH3COOH��0.1 mol•L-1��ˮ�������ϣ�pH=7���� c��NH4+��=c��CH3COO-��=c��H+��=c��OH-�� | |
| B�� | 0.1 mol•L-1HCl��Һ��0.2 mol•L-1��ˮ�������ϣ�pH��7���� c��NH4+����c��Cl-����c��NH3•H2O����c��OH-�� | |
| C�� | 0.1 mol•L-1CH3COONa��Һ��0.1 mol•L-1CaCl2��Һ�������ϣ� c��Na+��+c��Ca2+��=c��CH3COO-��+c��CH3COOH��+2c��Cl-�� | |
| D�� | 0.1 mol•L-1Na2CO3��Һ��0.1 mol•L-1 NaHCO3��Һ�������ϣ� c��HCO3-����0.05 mol•L-1��c��CO32-����c��OH-�� |
20�������Ҵ������в����ڵ��������ǣ�������
| A�� | ��� | B�� | ���Լ� | C�� | �Ǽ��Լ� | D�� | ���Ӽ� |
16�������й�ʵ��װ�ý��е���Ӧʵ�飬�ܴﵽʵ��Ŀ���ǣ�������

| A�� | ��ͼ1��ʾװ����ȡ����İ��� | |
| B�� | ͼ2װ�ÿ�������ȡ����������CO2���� | |
| C�� | ��ͼ3��ʾװ�ý���Һ���ݵ�100 mL | |
| D�� | ͼ4װ�ÿ����ڱȽ�̼���ƺ�̼�����Ƶ����ȶ��ԣ����Թ�A��װ̼���ƹ��壬С�Թ�B��װ̼�����ƹ��� |