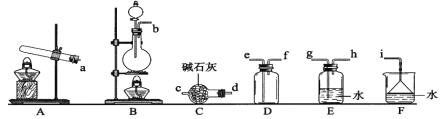
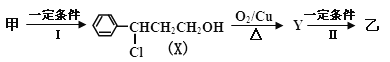题目内容
【题目】有A、B、C、D、E五种元素,其中A、B、C属于同一周期,A原子最外层p轨道的电子数等于次外层的电子总数,B元素可分别与A、C、D、E生成RB2型化合物,并知在DB2和EB2中,D与B的质量比为7∶8,E与B的质量比为1∶1。根据以上条件,回答下列问题:
(1)画出C的原子结构示意图:________。
(2)写出D原子的外围电子排布式:________。
(3)写出A元素单质在B中完全燃烧的化学方程式:______________。
(4)指出E元素在元素周期表中的位置:____________。
(5)比较A、B、C三种元素的第一电离能的大小顺序:________________(按由大到小的顺序排列,用元素符号表示)。
(6)比较元素D和E的电负性的相对大小:__________。
【答案】 ![]() 3s23p2 C+O2
3s23p2 C+O2![]() CO2 第三周期第ⅥA族 N>O>C S>Si
CO2 第三周期第ⅥA族 N>O>C S>Si
【解析】考查元素周期表和元素周期律的应用,A原子最外层p轨道的电子数等于次外层的电子总数,p能级上最多容纳6个电子,应为第二周期元素,电子排布式为1s22s22p2,即A为C,B与A形成AB2型化合物,且A、B、C属于同一周期,则B为O,C为N,DB2中D与B质量比为7:8,则有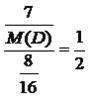 ,解得M(D)=28g·mol-1,即D为Si,同理推出E为S,(1)N的原子结构示意图为
,解得M(D)=28g·mol-1,即D为Si,同理推出E为S,(1)N的原子结构示意图为![]() ;(2)Si属于主族元素,Si的外围电子指的是最外层电子,即外围电子排布式为3s23p2 ;(3)C在氧气中完全燃烧,反应方程式为C+O2
;(2)Si属于主族元素,Si的外围电子指的是最外层电子,即外围电子排布式为3s23p2 ;(3)C在氧气中完全燃烧,反应方程式为C+O2![]() CO2 ;(4)S位于第三周期VIA族;(5)同周期从左向右第一电离能增大,但IIIA>IIA、VA>VIA,因此三种元素的第一电离能的顺序是N>O>C;(6)S和Si属于同周期,从左向右电负性增大,即S>Si。
CO2 ;(4)S位于第三周期VIA族;(5)同周期从左向右第一电离能增大,但IIIA>IIA、VA>VIA,因此三种元素的第一电离能的顺序是N>O>C;(6)S和Si属于同周期,从左向右电负性增大,即S>Si。

 提分百分百检测卷系列答案
提分百分百检测卷系列答案 宝贝计划期末冲刺夺100分系列答案
宝贝计划期末冲刺夺100分系列答案 能考试全能100分系列答案
能考试全能100分系列答案