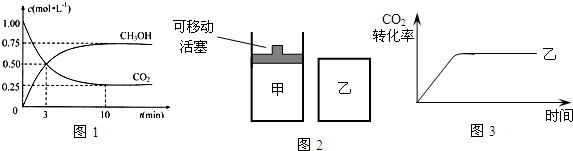
��Ŀ����
��̼��������ֱ�Ӻϳ��Ҵ�ȼ���ѽ�����ģ��������ͼ���ɶ�����̼�ϳ��Ҵ��ļ������̣�
���ճ���ʢ�б���̼�����Һ���Ѻ��ж�����̼�Ŀ����������ճ��У����ճ��з�ӦҺ����ֽ�غ���ֽ����ͨ�����ˮ�������Ѷ�����̼����Һ����ȡ�������ںϳ����к���������ѧ��Ӧʹ֮��Ϊ������ȼ���Ҵ����ش��������⣺
��1��д�����ճ��з�Ӧ�����ӷ���ʽ______��
��2���ӷֽ����ѭ��ʹ�õ�������______��
��3����ҵ�ϻ���ȡ��CO��H2Ϊԭ�Ϻϳ��Ҵ����仯ѧ��Ӧ����ʽΪ��2CO��g��+4H2��g��?CH3CH2OH��g��+H2O��g��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=______��
��4������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH��ƽ�ⳣ��������CO��ȡCH3CH2OH�����CO2�Ʊ�CH3CH2OH���ŵ���______��д��һ�㼴�ɣ���
��5����֪��һ�������£�2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43kJ���������ڸ������£�
��д����ʾ�Ҵ�ȼ���ȵ��Ȼ�ѧ����ʽ______���������Ҵ���ȼ�ϣ�KOH������ʣ�����ȼ�ϵ�أ���д�������ĵ缫����ʽ��______��
��6����һ��ѹǿ�£������CO2��ȡCH3CH2OH��ʵ���������±���
���ݱ������ݷ�����
�¶����ߣ��÷�Ӧ��ƽ�ⳣ��Kֵ______��ѡ���������С�����䡱����
���ճ���ʢ�б���̼�����Һ���Ѻ��ж�����̼�Ŀ����������ճ��У����ճ��з�ӦҺ����ֽ�غ���ֽ����ͨ�����ˮ�������Ѷ�����̼����Һ����ȡ�������ںϳ����к���������ѧ��Ӧʹ֮��Ϊ������ȼ���Ҵ����ش��������⣺
��1��д�����ճ��з�Ӧ�����ӷ���ʽ______��
��2���ӷֽ����ѭ��ʹ�õ�������______��
��3����ҵ�ϻ���ȡ��CO��H2Ϊԭ�Ϻϳ��Ҵ����仯ѧ��Ӧ����ʽΪ��2CO��g��+4H2��g��?CH3CH2OH��g��+H2O��g��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=______��
��4������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH��ƽ�ⳣ��������CO��ȡCH3CH2OH�����CO2�Ʊ�CH3CH2OH���ŵ���______��д��һ�㼴�ɣ���
��5����֪��һ�������£�2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43kJ���������ڸ������£�
��д����ʾ�Ҵ�ȼ���ȵ��Ȼ�ѧ����ʽ______���������Ҵ���ȼ�ϣ�KOH������ʣ�����ȼ�ϵ�أ���д�������ĵ缫����ʽ��______��
��6����һ��ѹǿ�£������CO2��ȡCH3CH2OH��ʵ���������±���
| �¶ȣ�K�� CO2ת����% n��H2��/n��CO2�� | 500 | 600 | 700 | 800 |
| 1.5 | 45 | 33 | 20 | 12 |
| 2.0 | 60 | 43 | 28 | 15 |
| 3.0 | 83 | 62 | 37 | 22 |
�¶����ߣ��÷�Ӧ��ƽ�ⳣ��Kֵ______��ѡ���������С�����䡱����

��1�����ճ���ʢ�б���̼�����Һ�����տ����еĶ�����̼��ת��ΪKHCO3����Ӧ���ӷ���ʽΪCO2+CO32-+H2O=2HCO3-��
�ʴ�Ϊ��CO2+CO32-+H2O=2HCO3-��
��2���ɹ�������ת����ϵ��֪���ֽ����KHO3�ֽ�����K2CO3��̼����ڱ�ѭ���������տ����ж�����̼��
�ʴ�Ϊ��K2CO3��
��3�����淴Ӧ�ﵽƽ��ʱ����������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ�dz�������K��ʾ����������л�ѧƽ�ⳣ����2CO��g��+4H2��g��?CH3CH2OH��g��+H2O��g����ƽ�ⳣ��k=
��
�ʴ�Ϊ��
��
��4������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH��ƽ�ⳣ��������CO��ȡCH3CH2OH��ԭ���нϴ��ת���ʣ�̼�����Һ���տ����еĶ�����̼��ת��ΪKHO3��KHO3�ֽ�����CO2��CO2ԭ���ã�
�ʴ�Ϊ��ԭ���нϴ��ת���ʣ�CO2ԭ���ã�
��5����2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43kJ��������46g�Ҵ���ȫȼ������Һ̬ˮ�ų�1366.89kJ�������Ȼ�ѧ����ʽ��д����д������ע���ʾۼ�״̬�Ͷ�Ӧ��Ӧ�ʱ䣬��Ӧ���Ȼ�ѧ����ʽΪ��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l������H=-1366.89kJ/mol��
�ʴ�Ϊ��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l������H=-1366.89kJ/mol��
�����Ҵ���ȼ�ϣ�KOH������ʣ�����ȼ�ϵ�أ�ȼ���Ҵ��ڸ���ʧ���ӷ���������Ӧ���ڼ���Һ������̼���Σ��缫��ӦΪ��C2H5OH-12e-+16OH-=2CO32-+11H2O��
�ʴ�Ϊ��C2H5OH-12e-+16OH-=2CO32-+11H2O��
��6���ɱ������ݿ�֪��n��H2��/n��CO2��һ������£��¶�Խ�ߣ�CO2ת����Խ�ͣ�˵�������¶�ƽ�����淴Ӧ�ƶ�������ƽ�ⳣ��k��С��
�ʴ�Ϊ����С��
�ʴ�Ϊ��CO2+CO32-+H2O=2HCO3-��
��2���ɹ�������ת����ϵ��֪���ֽ����KHO3�ֽ�����K2CO3��̼����ڱ�ѭ���������տ����ж�����̼��
�ʴ�Ϊ��K2CO3��
��3�����淴Ӧ�ﵽƽ��ʱ����������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ�dz�������K��ʾ����������л�ѧƽ�ⳣ����2CO��g��+4H2��g��?CH3CH2OH��g��+H2O��g����ƽ�ⳣ��k=
| c(H2O)c(CH3CH2OH) |
| c2(CO)c4(H2) |
�ʴ�Ϊ��
| c(H2O)c(CH3CH2OH) |
| c2(CO)c4(H2) |
��4������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH��ƽ�ⳣ��������CO��ȡCH3CH2OH��ԭ���нϴ��ת���ʣ�̼�����Һ���տ����еĶ�����̼��ת��ΪKHO3��KHO3�ֽ�����CO2��CO2ԭ���ã�
�ʴ�Ϊ��ԭ���нϴ��ת���ʣ�CO2ԭ���ã�
��5����2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43kJ��������46g�Ҵ���ȫȼ������Һ̬ˮ�ų�1366.89kJ�������Ȼ�ѧ����ʽ��д����д������ע���ʾۼ�״̬�Ͷ�Ӧ��Ӧ�ʱ䣬��Ӧ���Ȼ�ѧ����ʽΪ��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l������H=-1366.89kJ/mol��
�ʴ�Ϊ��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l������H=-1366.89kJ/mol��
�����Ҵ���ȼ�ϣ�KOH������ʣ�����ȼ�ϵ�أ�ȼ���Ҵ��ڸ���ʧ���ӷ���������Ӧ���ڼ���Һ������̼���Σ��缫��ӦΪ��C2H5OH-12e-+16OH-=2CO32-+11H2O��
�ʴ�Ϊ��C2H5OH-12e-+16OH-=2CO32-+11H2O��
��6���ɱ������ݿ�֪��n��H2��/n��CO2��һ������£��¶�Խ�ߣ�CO2ת����Խ�ͣ�˵�������¶�ƽ�����淴Ӧ�ƶ�������ƽ�ⳣ��k��С��
�ʴ�Ϊ����С��

��ϰ��ϵ�д�
�����Ŀ
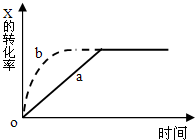


 ��������/n�������������]��ѹǿ�ı仯��ϵ�������������߷ֱ��ʾ��ϵѹǿΪ1.5MPa��2.5MPa��3.5MPa�������������Ӧ��ѹǿ��P���ף�=______��
��������/n�������������]��ѹǿ�ı仯��ϵ�������������߷ֱ��ʾ��ϵѹǿΪ1.5MPa��2.5MPa��3.5MPa�������������Ӧ��ѹǿ��P���ף�=______��