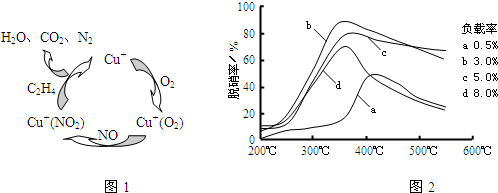
��Ŀ����
����������SO2����������������NOx���������ǻ�����ѧ�о����ȵ㡣
��1���������������Ļ���������___________________��
��2��һ��ѡ���Դ�������NO2����ԭ��Ϊ��6NO2��8NH3 7N2��12H2O
7N2��12H2O
�� ������Ӧ�б���ԭ��Ԫ����_________����Ԫ�ط��ţ�����Ӧ��ÿת��3mol���ӣ����ɱ�״����
N2�����Ϊ___________��
�� �����ٷɻ��ŷŵ�β����ƽ������NOx����Ҫ��Դ�������ƻ����������Ҫ����Ϊ��
��O3 O��O2 ��NO��O3��NO2��O2 ��NO2��O��NO��O2
O��O2 ��NO��O3��NO2��O2 ��NO2��O��NO��O2
������Ӧ��NOx�����������____________��
��3���±��г���2��ȼú����������ԭ����
��1���������������Ļ���������___________________��
��2��һ��ѡ���Դ�������NO2����ԭ��Ϊ��6NO2��8NH3
 7N2��12H2O
7N2��12H2O �� ������Ӧ�б���ԭ��Ԫ����_________����Ԫ�ط��ţ�����Ӧ��ÿת��3mol���ӣ����ɱ�״����
N2�����Ϊ___________��
�� �����ٷɻ��ŷŵ�β����ƽ������NOx����Ҫ��Դ�������ƻ����������Ҫ����Ϊ��
��O3
 O��O2 ��NO��O3��NO2��O2 ��NO2��O��NO��O2
O��O2 ��NO��O3��NO2��O2 ��NO2��O��NO��O2 ������Ӧ��NOx�����������____________��
��3���±��г���2��ȼú����������ԭ����

�ٷ��������ð�ˮ����ȼú�����е�SO2ת��ΪNH4HSO3����������SO2��_______���ʣ�ѡ����ĸ��ţ�
A��Ư���� B�������� C����ԭ�� D������������
�ڷ�������Ҫ���������з�Ӧ��
2CO(g)+SO2(g)=== S(g)+2CO2(g) ��H1��8��0kJ��mol-1
2H2(g) +SO2(g)=== S(g)+2H2O(g) ��H2��90��4 kJ��mol-1
2CO(g)+O2(g) === 2CO2(g) ��H3����566��0 kJ��mol-1
��д��S(g)��O2��Ӧ����SO2���Ȼ�ѧ����ʽ__________________________
A��Ư���� B�������� C����ԭ�� D������������
�ڷ�������Ҫ���������з�Ӧ��
2CO(g)+SO2(g)=== S(g)+2CO2(g) ��H1��8��0kJ��mol-1
2H2(g) +SO2(g)=== S(g)+2H2O(g) ��H2��90��4 kJ��mol-1
2CO(g)+O2(g) === 2CO2(g) ��H3����566��0 kJ��mol-1
��д��S(g)��O2��Ӧ����SO2���Ȼ�ѧ����ʽ__________________________
��1����ֹ����ķ���
��2����N��19.6L���ڴ���
��3����D����S(g)+O2(g)==SO2(g) ��H=-574.0kJ��mol-1
��2����N��19.6L���ڴ���
��3����D����S(g)+O2(g)==SO2(g) ��H=-574.0kJ��mol-1

��ϰ��ϵ�д�
�����Ŀ
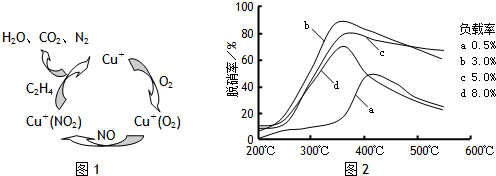
 ��X��Y��Z�Ƕ�����Ԫ�ص����ֳ��������X��ˮ��Ӧ������һ�־��л�ԭ�ԵIJ��ȶ��Ķ�Ԫ�ᣬ����Ļ�ѧʽ��
��X��Y��Z�Ƕ�����Ԫ�ص����ֳ��������X��ˮ��Ӧ������һ�־��л�ԭ�ԵIJ��ȶ��Ķ�Ԫ�ᣬ����Ļ�ѧʽ��