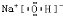��Ŀ����
 ��X��Y��Z�Ƕ�����Ԫ�ص����ֳ��������X��ˮ��Ӧ������һ�־��л�ԭ�ԵIJ��ȶ��Ķ�Ԫ�ᣬ����Ļ�ѧʽ��
��X��Y��Z�Ƕ�����Ԫ�ص����ֳ��������X��ˮ��Ӧ������һ�־��л�ԭ�ԵIJ��ȶ��Ķ�Ԫ�ᣬ����Ļ�ѧʽ��H2SO3
H2SO3
��Y��X���Ԫ����ͬ��Y��ˮ��Ӧ����M������M��Ũ��Һ��3.2g Cu�ڼ��������³�ַ�Ӧ�����б���ԭ��M�����ʵ���Ϊ0.05mol
0.05mol
����̬Z��Ӧ�����˹����꣬0.5mol?L-1Z��NaOH��Ӧ���õ�����ˮ��Һ�У�����Ũ�ȴӴ�С��˳��ΪC��Na+����C��CO32- ����C��OH-����C��HCO3-����C��H+��
C��Na+����C��CO32- ����C��OH-����C��HCO3-����C��H+��
����N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע������H2��O2��������Na2CO3��ɵ�ȼ�ϵ�أ�����YΪCO2�����õ�ⷨ�Ʊ�N2O5���ܵķ�Ӧ����ʽΪ��N2O4+2HNO3=2N2O5+H2��װ����ͼ��ʾ��
д��ʯīI�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ
H2+CO32--2e-=CO2+H2O
H2+CO32--2e-=CO2+H2O
���ڵ���������N2O5�ĵ缫��ӦʽΪ
N2O4+2HNO3-2e-=2N2O5+2H+
N2O4+2HNO3-2e-=2N2O5+2H+
��������������SO2����������������NOx���������ǻ�����ѧ�о����ȵ㣮
��1���������������Ի���������
��ֹ���ꡢ�⻯ѧ�����ķ���
��ֹ���ꡢ�⻯ѧ�����ķ���
����2��Ŀǰ����ѧ�������о�һ������ϩ��Ϊ��ԭ����������NO��ԭ��������������ʾ��ͼ��ͼ1��

���������¶ȡ������ʣ�����ɸ�д����������������Ĺ�ϵ��ͼ2��ʾ��
��д��������ԭ���ܷ�Ӧ�Ļ�ѧ����ʽ��
6NO+3O2+2C2H4
3N2+4CO2+4H2O
| ||
6NO+3O2+2C2H4
3N2+4CO2+4H2O
����Ϊ�ﵽ�������Ч����Ӧ��ȡ��������
| ||
350�桢������3%
350�桢������3%
�����������Ķ�Ԫ����H2SO4��H2SO3��H2CO3��H2SiO3�����ݶ�Ԫ���Ƿ��л�ԭ���ж�X���Ӷ�ȷ������Ļ�ѧʽ��
����������xȷ��Y��M�Ļ�ѧʽ������M��ͭ�ķ�Ӧ����ʽ���㱻��ԭ��M�����ʵ�����
���ݡ��������˹��������������ȷ��Z������Z���������Ʒ�Ӧ�������������ȷ����Һ�и����ӵ���Դ�С��
��ȼ��ԭ����У�������ȼ��ʧ���ӷ���������Ӧ���ȸ��ݻ��ϼ��ж�����N2O5�ĵ缫���ٸ������ӵķŵ�˳��д���缫��Ӧʽ��
��1��SO2�͵��������NOx���������������Ҫ���ʣ����������NOx��Ҳ������⻯ѧ������Ҫ���ʣ�
��2���ٸ���ͼ1ȷ����Ӧ������д����Ӧ����ʽ��
�������ʸߣ������ʵͣ����˵��¶ȣ�
����������xȷ��Y��M�Ļ�ѧʽ������M��ͭ�ķ�Ӧ����ʽ���㱻��ԭ��M�����ʵ�����
���ݡ��������˹��������������ȷ��Z������Z���������Ʒ�Ӧ�������������ȷ����Һ�и����ӵ���Դ�С��
��ȼ��ԭ����У�������ȼ��ʧ���ӷ���������Ӧ���ȸ��ݻ��ϼ��ж�����N2O5�ĵ缫���ٸ������ӵķŵ�˳��д���缫��Ӧʽ��
��1��SO2�͵��������NOx���������������Ҫ���ʣ����������NOx��Ҳ������⻯ѧ������Ҫ���ʣ�
��2���ٸ���ͼ1ȷ����Ӧ������д����Ӧ����ʽ��
�������ʸߣ������ʵͣ����˵��¶ȣ�
����⣺�����Ķ�Ԫ��������H2SO4��H2SO3��H2CO3��H2SiO3�����л�ԭ�ԵĶ�Ԫ������Ϊ�����ᣬ��ѧʽ��H2SO3������XΪ��������
Y��X���Ԫ����ͬ������Y����������M�����ᣬŨ�����ͭ��Ӧ�ķ���ʽΪ
Cu+H2SO4��Ũ��+H2SO4��Ũ�� ������ԭ���ᣩ
CuSO4+SO2��+2H2O��
64g 1mol
3.2g 0.05mol
���Ա���ԭ������Ϊ0.05mol��
��̬��Ӧ�����˹������������Ϊ������̼������Z�Ƕ�����̼��������̼��NaOH��Ӧ���õ�����Ϊ̼���ƣ�̼������ǿ�������Σ������ܵ���Ҳ��ˮ�⣬ֻ�ǵ���̶ȴ���ˮ��̶ȣ�����̼�������Ũ�ȴ���̼���������Ũ�ȣ�̼���Ƕ�Ԫ���ᣬ����̼��������ܷ�������ˮ�⣬��һ��ˮ��̶ȴ��ڵڶ���ˮ��̶ȣ�̼�������ÿ�������������������ɣ��������������ӵ�Ũ�ȴ���̼��������ӵ�Ũ�ȣ�̼������ǿ�������Σ���ˮ��Һ�ʼ��ԣ���������������Ũ�ȴ���������Ũ�ȣ�������Һ�����ӵ�Ũ�ȴ�СΪC��Na+����C��CO32- ����C��OH-����C��HCO3-����C��H+����
�ʴ�Ϊ��H2SO3��0.05mol��C��Na+����C��CO32- ����C��OH-����C��HCO3-����C��H+����
��ȼ��ԭ����У�������ȼ��ʧ���Ӻ�̼������ӷ�Ӧ���ɶ�����̼��ˮ���缫��ӦʽΪH2+CO32--2e-=CO2+H2O��
N2O5�е�Ԫ�صĻ��ϼ���+5�ۣ��������е�Ԫ��Ҳ��+5�ۣ����Ӧ�����������N2O5�����������������ɣ��ݵ缫��Ӧ���ӷŵ�˳���֪����������2H++2e-=H2���ķ�Ӧ��������ΪN2O4+2HNO3-2e-=2N2O5+2H+��
�ʴ�Ϊ��H2+CO32--2e-=CO2+H2O��N2O4+2HNO3-2e-=2N2O5+2H+��
��1����SO2�͵��������NOx���������������Ҫ���ʣ������������������Ļ��������Ƿ�ֹ���ꡢ�⻯ѧ�����ķ������ʴ�Ϊ����ֹ���ꡢ�⻯ѧ�����ķ�����
��2������ͼ1֪����Ӧ��Ϊһ����������������ϩ��ͭΪ������������Ϊ������������̼��ˮ����Ӧ����ʽΪ6NO+3O2+2C2H4
3N2+4CO2+4H2O��
�ʴ�Ϊ��6NO+3O2+2C2H4
3N2+4CO2+4H2O����
�����������ʸߣ������ʵͣ����˵��¶ȣ���ͼ��֪���ʺ�����Ϊ350�桢������3%���ʴ�Ϊ��350�桢������3%��
Y��X���Ԫ����ͬ������Y����������M�����ᣬŨ�����ͭ��Ӧ�ķ���ʽΪ
Cu+H2SO4��Ũ��+H2SO4��Ũ�� ������ԭ���ᣩ
| ||
64g 1mol
3.2g 0.05mol
���Ա���ԭ������Ϊ0.05mol��
��̬��Ӧ�����˹������������Ϊ������̼������Z�Ƕ�����̼��������̼��NaOH��Ӧ���õ�����Ϊ̼���ƣ�̼������ǿ�������Σ������ܵ���Ҳ��ˮ�⣬ֻ�ǵ���̶ȴ���ˮ��̶ȣ�����̼�������Ũ�ȴ���̼���������Ũ�ȣ�̼���Ƕ�Ԫ���ᣬ����̼��������ܷ�������ˮ�⣬��һ��ˮ��̶ȴ��ڵڶ���ˮ��̶ȣ�̼�������ÿ�������������������ɣ��������������ӵ�Ũ�ȴ���̼��������ӵ�Ũ�ȣ�̼������ǿ�������Σ���ˮ��Һ�ʼ��ԣ���������������Ũ�ȴ���������Ũ�ȣ�������Һ�����ӵ�Ũ�ȴ�СΪC��Na+����C��CO32- ����C��OH-����C��HCO3-����C��H+����
�ʴ�Ϊ��H2SO3��0.05mol��C��Na+����C��CO32- ����C��OH-����C��HCO3-����C��H+����
��ȼ��ԭ����У�������ȼ��ʧ���Ӻ�̼������ӷ�Ӧ���ɶ�����̼��ˮ���缫��ӦʽΪH2+CO32--2e-=CO2+H2O��
N2O5�е�Ԫ�صĻ��ϼ���+5�ۣ��������е�Ԫ��Ҳ��+5�ۣ����Ӧ�����������N2O5�����������������ɣ��ݵ缫��Ӧ���ӷŵ�˳���֪����������2H++2e-=H2���ķ�Ӧ��������ΪN2O4+2HNO3-2e-=2N2O5+2H+��
�ʴ�Ϊ��H2+CO32--2e-=CO2+H2O��N2O4+2HNO3-2e-=2N2O5+2H+��
��1����SO2�͵��������NOx���������������Ҫ���ʣ������������������Ļ��������Ƿ�ֹ���ꡢ�⻯ѧ�����ķ������ʴ�Ϊ����ֹ���ꡢ�⻯ѧ�����ķ�����
��2������ͼ1֪����Ӧ��Ϊһ����������������ϩ��ͭΪ������������Ϊ������������̼��ˮ����Ӧ����ʽΪ6NO+3O2+2C2H4
| ||
�ʴ�Ϊ��6NO+3O2+2C2H4
| ||
�����������ʸߣ������ʵͣ����˵��¶ȣ���ͼ��֪���ʺ�����Ϊ350�桢������3%���ʴ�Ϊ��350�桢������3%��
���������⿼����ԭ��غ͵��صĹ���ԭ����ͼ��ķ������ѶȽϴ��״���Ϊ���⣬����ݻ��ϼ۵ı仯ȷ������N2O5�ĵ缫���������ӵķŵ�˳��д����Ӧ�ĵ缫��Ӧʽ��

��ϰ��ϵ�д�
�����Ŀ