��Ŀ����
��һ����ɱ���ܱ������У�����һ������X��Y��������ӦmX(g) nY(g) ��H=QkJ/mol����Ӧ�ﵽƽ��ʱ��Y�����ʵ���Ũ�����¶ȡ���������Ĺ�ϵ���±���ʾ������˵����ȷ����
nY(g) ��H=QkJ/mol����Ӧ�ﵽƽ��ʱ��Y�����ʵ���Ũ�����¶ȡ���������Ĺ�ϵ���±���ʾ������˵����ȷ����
A��m��n B���¶Ȳ��䣬ѹǿ����Y��������������
C��Q��0 D��������䣬�¶����ߣ�ƽ�����淴Ӧ�����ƶ�
 nY(g) ��H=QkJ/mol����Ӧ�ﵽƽ��ʱ��Y�����ʵ���Ũ�����¶ȡ���������Ĺ�ϵ���±���ʾ������˵����ȷ����
nY(g) ��H=QkJ/mol����Ӧ�ﵽƽ��ʱ��Y�����ʵ���Ũ�����¶ȡ���������Ĺ�ϵ���±���ʾ������˵����ȷ����| �������/L c(Y)/mol/L �¶�/�� | 1 | 2 | 3 |
| 100 | 1.00 | 0.75 | 0.53 |
| 200 | 1.20 | 0.90 | 0.63 |
| 300 | 1.30 | 1.00 | 0.70 |
A��m��n B���¶Ȳ��䣬ѹǿ����Y��������������
C��Q��0 D��������䣬�¶����ߣ�ƽ�����淴Ӧ�����ƶ�
B
�����������ͼ�п��Կ��������¶Ȳ���������£����������ƽ�������ƶ��������������������ķ���Ҳ����˵��m��n ��A�������������������£������¶ȣ�ƽ��Ҳ�������ƶ��������������ȵķ�������У�Q��0��C��D����ֻ��B��ȷ��

��ϰ��ϵ�д�
 �������ϵ�д�
�������ϵ�д�
�����Ŀ
 2NH3(g)��CO2(g) ��H =" a" kJ/mol
2NH3(g)��CO2(g) ��H =" a" kJ/mol  pC�ﵽƽ�⣬����
pC�ﵽƽ�⣬���� xC(g),��ƽ���C��ƽ�������е��������Ϊw����ά���¶Ȳ��䣬��1.2molA��0.4molB��0.6molCΪ��ʼ���ʣ���ƽ���ѹǿ���䣬C�����������Ϊw����x��ֵΪ
xC(g),��ƽ���C��ƽ�������е��������Ϊw����ά���¶Ȳ��䣬��1.2molA��0.4molB��0.6molCΪ��ʼ���ʣ���ƽ���ѹǿ���䣬C�����������Ϊw����x��ֵΪ C(g)��ƽ��ʱC ���������Ϊ40�G��
C(g)��ƽ��ʱC ���������Ϊ40�G�� Y(g)+Z(s)��������˵����Ӧ�ﵽƽ���־����( )
Y(g)+Z(s)��������˵����Ӧ�ﵽƽ���־����( ) 4NO(g)+6H2O(g)��������������ȷ����
4NO(g)+6H2O(g)��������������ȷ���� H2(g)+CO2(g)ƽ�ⳣ�����¶ȵı仯���±���
H2(g)+CO2(g)ƽ�ⳣ�����¶ȵı仯���±��� ��һ���ؼ��IJ��衣
��һ���ؼ��IJ��衣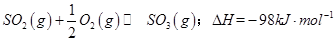 ����ʼʱ��100L���ܱ������м���4.0molSO2(g)��10.0molO2������Ӧ�ﵽƽ��ʱ���ų�����196kJ�����¶���ƽ�ⳣ��K=____________��
����ʼʱ��100L���ܱ������м���4.0molSO2(g)��10.0molO2������Ӧ�ﵽƽ��ʱ���ų�����196kJ�����¶���ƽ�ⳣ��K=____________��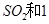 mol
mol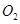 ,������Ӧ:
,������Ӧ: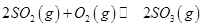 ,��ƽ���ı�����������
,��ƽ���ı�����������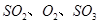 ����ƽ��Ũ�ȶ���ԭ���������____________(����ĸ)��
����ƽ��Ũ�ȶ���ԭ���������____________(����ĸ)��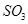

 ��������Һ���������̹�ת����0.1mol����ʱ������������SO2�����Ϊ����״����Ϊ_________������Һ��pH="__________" (������Һ����仯)��
��������Һ���������̹�ת����0.1mol����ʱ������������SO2�����Ϊ����״����Ϊ_________������Һ��pH="__________" (������Һ����仯)��