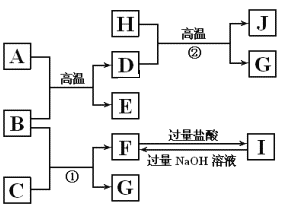
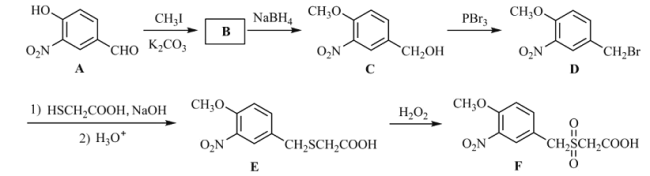
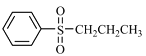
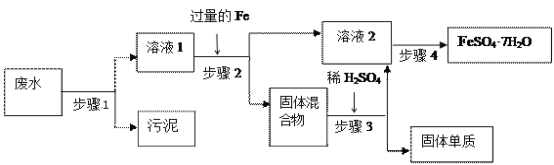
��Ŀ����
����Ŀ������������Һ�Ͷ�������������(C3N3O3Cl2Na)���dz��õ�ɱ���������� NaClO�������Ʊ���������������.
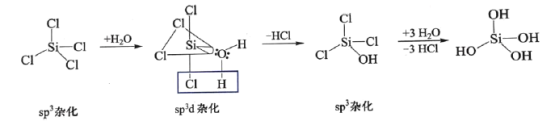
(1)NaClO��Һ���ɵ����½�Cl2����ͨ��NaOH��Һ�ж��Ƶá��Ʊ� NaClO�����ӷ���ʽΪ__________�����ڻ���ɱ��������NaClO��Һ��ϡ�Ͳ���ʱʹ�ã����ڿ����б�¶ʱ������Ҽ��⣬���ᵼ���������ü�������ԭ����__________________��
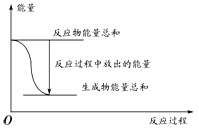
(2)������������������ƷҪ����Ч�ȴ���60%��ͨ������ʵ�������������������Ʒ�Ƿ�ﵽ����Ʒ����ʵ����ԭ��Ϊ![]()
![]()
![]()
ȷ��ȡ1.1200g��Ʒ��������ƿ���250.0mL��Һ��ȡ25.00mL������Һ�ڵ���ƿ�У���������ϡ�������KI��Һ���ܷ��ڰ�������5min����![]() Na2S2O3����Һ�ζ�����Һ����ɫ���������ָʾ�������ζ����յ㣬����Na2S2O3��Һ20.00mL��
Na2S2O3����Һ�ζ�����Һ����ɫ���������ָʾ�������ζ����յ㣬����Na2S2O3��Һ20.00mL��
��ͨ�������жϸ���Ʒ�Ƿ�Ϊ����Ʒ_______��(д��������̣�![]() )
)
�����ڼ���м���ϡ����������٣���������Ʒ����Ч�Ȳⶨֵ____________(�ƫ�ߡ���ƫ�͡�)��
���𰸡�![]() NaClO��Һ���տ����е�CO2�����HClO��HClO����ֽ�
NaClO��Һ���տ����е�CO2�����HClO��HClO����ֽ� ![]()
��������ת���͵��ӵ�ʧ�غ��ϵ��![]()
��![]()
��Ԫ�ص�����:
![]()
����Ʒ����Ч��Ϊ��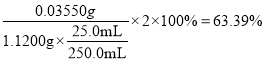
����Ʒ����Ч�ȴ���60%���ʸ���ƷΪ����Ʒ ƫ��
��������
(1) �������֪������ͨ�����������в����������ƣ�ͬʱ�����Ȼ��ƣ���Ӧ�����ӷ���ʽΪ��![]() ������������Һ���ڱ�¶�ڿ����л����տ����еĶ�����̼���壬����������Ա�̼��������˴������ƿ����������̼��ˮ�з�Ӧ����HClO��HClO���в��ȶ��ԣ������Ȼ���������»ᷢ���ֽⷴӦ������HCl��O2���Ӷ��Ǵ�������ʧЧ���ʴ�Ϊ��
������������Һ���ڱ�¶�ڿ����л����տ����еĶ�����̼���壬����������Ա�̼��������˴������ƿ����������̼��ˮ�з�Ӧ����HClO��HClO���в��ȶ��ԣ������Ȼ���������»ᷢ���ֽⷴӦ������HCl��O2���Ӷ��Ǵ�������ʧЧ���ʴ�Ϊ��![]() ��NaClO��Һ���տ����е�CO2�����HClO��HClO����ֽ⣻
��NaClO��Һ���տ����е�CO2�����HClO��HClO����ֽ⣻
(2) �������з�Ӧ��֪��![]() ��������������HClO��HClO���������Ӳ����ⵥ�ʣ��ⵥ��������������Ƶζ�����Ϸ�Ӧת��ȷ������֮��Ĺ�ϵΪ��
��������������HClO��HClO���������Ӳ����ⵥ�ʣ��ⵥ��������������Ƶζ�����Ϸ�Ӧת��ȷ������֮��Ĺ�ϵΪ��![]() ��
�� ![]() ����������ת���͵��ӵ�ʧ�غ��ϵ����n(Cl)=0.5
����������ת���͵��ӵ�ʧ�غ��ϵ����n(Cl)=0.5![]() =
=![]() ��
��
��Ԫ�ص�������m(Cl)= ![]() =0.03550g������Ʒ�е���Ч��Ϊ��
=0.03550g������Ʒ�е���Ч��Ϊ�� =63.39%��
=63.39%��
����Ʒ�е���Ч�ȴ���60%���ʸ���ƷΪ����Ʒ
�ʴ�Ϊ��n(S2O![]() )=
)=![]() ����������ת���͵��ӵ�ʧ�غ��ϵ��
����������ת���͵��ӵ�ʧ�غ��ϵ��![]() ����n(Cl)=0.5
����n(Cl)=0.5![]() =
=![]() ��
��
��Ԫ�ص�������m(Cl)= ![]() =0.03550g������Ʒ�е���Ч��Ϊ��
=0.03550g������Ʒ�е���Ч��Ϊ�� =63.39%��
=63.39%��
����Ʒ�е���Ч�ȴ���60%���ʸ���ƷΪ����Ʒ
�����������������٣����·�Ӧ![]() ���ܳ�ֽ��У�������HClO����ƫ�ͣ����յ���ʵ���õ���Ч�Ⱥ�����ƫ�ͣ�
���ܳ�ֽ��У�������HClO����ƫ�ͣ����յ���ʵ���õ���Ч�Ⱥ�����ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�