��Ŀ����
����������Ӧ���Ȼ�ѧ����ʽ��
��I2(g) + H2(g)  ��2HI(g)��������H =��9.48 kJ��mol��1��
��2HI(g)��������H =��9.48 kJ��mol��1��
��I2(s) + H2(g)  ��2HI(g)���� �� ��H =" +26.48" kJ��mol��1
��2HI(g)���� �� ��H =" +26.48" kJ��mol��1
����˵����ȷ���ǣ� ��
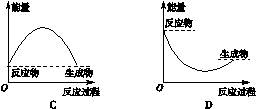
| A���ٵIJ���ȢڵIJ����ȶ� |
| B��I2(s) = I2(g)��������H=" +17.00" kJ��mol��1 |
| C���ڵķ�Ӧ���������Ȣٵķ�Ӧ���������� |
| D��1mol I2(g)��ͨ��1 mol H2(g)��������Ӧʱ����9.48 kJ |
C
��������������ٵIJ���͢ڵIJ��ﶼ��HI���ȶ�����ͬ��A����ȷ���ؼ���˹���ɿ�֪���ڣ��ټ��õ�I2(s)��I2(g)����H��26.48kJ/mol��9.48kJ/mol����35.96kJ/mol��ѡ��B����ȷ������������������̬�ĵ����������Ԣڵķ�Ӧ���������Ȣٵķ�Ӧ���������ͣ�C��ȷ���÷�Ӧ�ǿ��淴Ӧ����1mol I2(g)��ͨ��1 mol H2(g)��������Ӧʱ����һ��С��9.48kJ��D����ȷ����ѡC��
���㣺���鷴Ӧ�ȵļ���ͷ�Ӧ�ȵ��й�Ӧ��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣��Ҫ�ǿ���ѧ���Է�Ӧ�Ⱥ����Լ����ø�˹���ɼ��㷴Ӧ�ȵ��˽����������ּ�ڹ���ѧ���Ļ��������ѧ����Ӧ��������ѧϰЧ�ʡ�

��Դ�ǵ�����ᷢչ������֧��֮һ����ר�����������ܹ�����̫����ʹȼ��ȼ�ղ����CO2��H2O��N2��������ϣ���ͼ�������Խ�Լȼ�ϣ�������ԴΣ�����ڴ˹��������ѭ����̫��������ת��Ϊ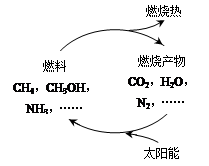
| A����ѧ�� | B������ |
| C�������� | D������ |
����˵���У���ȷ���� �� ��
| A�������£�pH=5.6��NaHSO3��Һ��c( SO32-)-c( H2SO3)= 10-5.6-10-8.4 |
| B��ͬ��ͬѹ�£���ӦH2(g)��Cl2(g)��2HCl(g)�ڹ��պ͵�ȼ�����µĦ�H��ͬ |
| C�����ȷ�Ӧֻ���ڼ��������²��ܷ�������ˮ�����Ȳ����� |
| D�������İ�ˮ�����ᷴӦ������Һ������Ϊ���ԣ�������Ϊ���� |
��ѧ��ӦH2��Cl2===2HCl�������仯��ͼ��ʾ��������˵����ȷ����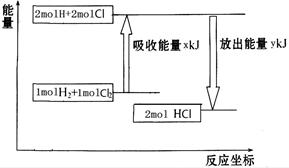
| A���÷�Ӧ�Ƿ��ȷ�Ӧ |
| B������1 mol H��H����1 mol Cl��Cl ���ų�x kJ���� |
| C������1 mol H��Cl����Ҫ����y kJ������ |
| D��2 mol HCl������������1 mol H2��1 molCl2�������� |
����˵���в���ȷ����
| A����ú�͡�������Ƚϣ���Ȼ����һ�ֱȽ����Ļ�ʯȼ�� |
| B���Ҵ���һ�ֿ�������Դ����Ϊȼ�ϵ��ŵ������ȫȼ�յIJ��ﲻ��Ⱦ���� |
| C������̫���ܡ����ܺ����ܵ���Դ�����ʯ��Դ����Ч���ƿ������� |
| D��ú��������Һ���������Ի�ýྻȼ�ϣ�������ȼ�ջ�ų���������� |
CO�dz����Ļ�ѧ���ʣ��ڹ�ҵ��������;�ܹ㷺��
��1�� ��֪��ijЩ��Ӧ���Ȼ�ѧ����ʽ���£�
2H2��g��+SO2��g��=S��g��+2H2O��g�� ��H��+90.4kJ��mol��1
2CO��g��+O2��g��=2CO2��g�� ��H��-556.0kJ��mol��1
2H2��g��+O2��g��=2H2O��g�� ��H��-483.6kJ��mol��1
��д����CO��ȥ������SO2������S��g����CO2�Ȼ�ѧ����ʽ
��2�� ijȼ�ϵ����COΪȼ�ϣ��Կ���Ϊ��������������̬��K2CO3Ϊ����ʣ���д����ȼ�ϵ�������ĵ缫��Ӧʽ ��
��3����ij�¶��¡��ݻ���Ϊ2L�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��º��ݣ�ʹ֮������Ӧ��2H2��g����CO��g�� CH3OH��g������H����dJ��mol��1��d��0������ʼͶ����������ﵽƽ��ʱ���й��������£�
CH3OH��g������H����dJ��mol��1��d��0������ʼͶ����������ﵽƽ��ʱ���й��������£�
| ʵ�� | �� | �� | �� |
| ��ʼͶ�� | 2 molH2��1 molCO | 1 mol CH3OH | 4 molH2��2 molCO |
| ƽ��ʱn��CH3OH �� | 0.5mol | n2 | n3 |
| ��Ӧ�������仯 | �ų�Q1kJ | ����Q2kJ | �ų�Q3kJ |
| ��ϵ��ѹǿ | P1 | P2 | P3 |
| ��Ӧ���ת���� | ��1 | ��2 | ��3 |
�����������еķ�Ӧ�ֱ��ƽ��ʱ�������ݹ�ϵ��ȷ���� ������ţ���
A����1����2��1 B��Q1��Q2��d
C����3����1 D��P3>2P1��2P2
E��n2��n3��1.0mol F��Q3��2Q1
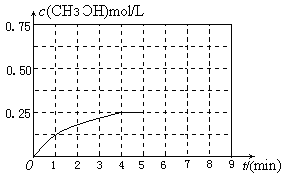
���������������������£�������������ϵ���ѹ����1L�����ڵ�8min�ﵽ�µ�ƽ�⣬�ӿ�ʼ����ƽ��ʱH2��ת����Ϊ65.5%��������ͼ�л�����5min ����ƽ��ʱCH3OH�����ʵ���Ũ�ȵı仯���ߡ�
��4��ʵ���ҳ��ü��ᣨһԪ�ᣩ���Ʊ�CO����֪25��ʱ��0.l mol/L����( HCOOH)��Һ��0.l mo1/L������Һ��pH�ֱ�Ϊ2.3��2.9��������ͬ���ʵ���Ũ�ȵ�����������Һ����HCOONa��Һ ��CH3COONa��Һ��Na2CO3��NaHCO3��Һ����pH�ɴ�С��˳���� ____����д��Һ��ţ�������0.l mo1/L HCOOH��Һ��0.l mo1/LHCOONa�������Ϻ����Һ������ȷ���� ____��
a��c(HCOO��)��c(HCOOH)��c��Na+����c��H+��
b��c(HCOO��)+c(HCOOH)=" 0.2" mo1/L
c��c(HCOO��)+2c(OH��)=c��HCOOH��+2c��H+��
d��c(HCOO��) ��c��Na+����c��H+����c(OH��)

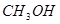 ��ԭ�ϡ���֪��
��ԭ�ϡ���֪��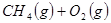
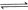
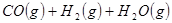
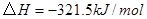
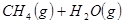
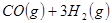
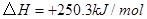
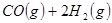
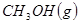
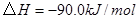
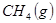 ��
��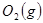 ��Ӧ����
��Ӧ����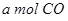 ��
��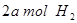 ���ڲ�ͬѹǿ�ºϳɼ״���
���ڲ�ͬѹǿ�ºϳɼ״��� ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��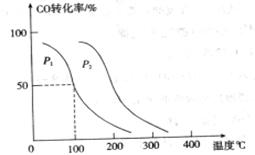
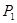
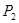 ���<������>����=����
���<������>����=����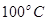 ��
��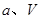 �Ĵ���ʽ��ʾ����
�Ĵ���ʽ��ʾ����