��Ŀ����
����Ŀ���ʻ���COS����ȫ����ѭ������Ҫ�м��壬Ҳ���л��ϳ��е���Ҫԭ�ϣ��ǻ�ѧ��������Ҫ���о�������֪��
��COS��g����H2��g��![]() H2S��g����CO��g�� ��Hl����17kJ��mol��
H2S��g����CO��g�� ��Hl����17kJ��mol��
��COS��g����H2O��g��![]() H2S��g����CO2��g�� ��H2����35kJ��mol��
H2S��g����CO2��g�� ��H2����35kJ��mol��
�ش��������⣺
��1����ӦCO��g����H2O��g��![]() H2��g����CO2��g���ġ�H��________��
H2��g����CO2��g���ġ�H��________��

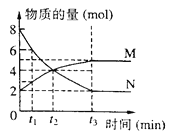
��2���ڳ��д����ĺ�ѹ�ܱ������н��з�ӦI������ʼ�����n��COS����n��H2����m����ͬʱ���ڲ��COSת������m���¶ȣ�T���Ĺ�ϵ��ͼ��ʾ��
��m1________m2�����������
���¶ȸ���T0ʱ��COSת���ʼ�С�Ŀ���ԭ��Ϊ_________��
A���и���Ӧ������ B����Ӧ�ġ�H����
C���������Խ��͡� D���淴Ӧ��������ı�����������Ӧ��������ı���
��3���ں��¡������ܱ������У����з�ӦI������˵������˵����ӦI�Ѵﵽƽ��״̬����____��
A��c��H2S����c��CO�� B��v����H2����v����H2S��
C�������������ܶȱ��ֲ��� D�������л������ƽ��Ħ���������ֲ���
E��c��COS�����ֲ���
��4��ij�¶��£������Ϊ2 L�ĺ����ܱ�������ͨ��5 mol COS��g����5 molH2O��g����������Ӧ��5 min��Ӧ�ﵽƽ�⣬���COS��g����ת����Ϊ60����
�ٷ�Ӧ����ʼ��5 min�ڣ���H2SŨ�ȱ仯��ʾ��ƽ����Ӧ�ٶ�v��H2S����________��
�ڸ��¶��£�������Ӧ��ƽ�ⳣ��K��________��
������������ͬʱ������ʹ������Ӧ��COS��ƽ��ת������������ʹ��Ӧ���ʼӿ��������________��
A����С�����ݻ� B�������¶�
C����������� D������һ����H2O��g��
���𰸡�-18 kJ��mol �� ACD BE 0.3mol/(Lmin) 2.25 D
��������
��1�����ݸ�˹���ɷ�Ӧ��-��CO��g����H2O��g��![]() H2��g����CO2��g������H����35kJ��mol-����17kJ��mol��=-18 kJ��mol ���𰸣�-18 kJ��mol��
H2��g����CO2��g������H����35kJ��mol-����17kJ��mol��=-18 kJ��mol ���𰸣�-18 kJ��mol��
(2)����ͬ������, n(COS )/ n(H2)��С, COSת���ʼ�С,����m1<m2;��: <��
��Toǰ,�����¶�,����Ӧ���ʼӿ�, COSת��������;�¶ȸ���Toʱ,�����Ļ��Խ���,��������Ӧ,�淴Ӧ��������ı�����������Ӧ��������ı���,���п��ܵ���COSת���ʼ�С,�ʱ����¶���,��������,�������: ACD��
(3) A.c(H2S) =c(CO) ,û��ָ����Ӧ���ʽ��еķ���,���ж���Ӧ�Ѵﵽƽ��״̬,A����; B.��(H2) =V��(H2S) ,ָ�����淴Ӧ���ʵķ���,����������֮�Ⱥ�ϵ��������,�����ж���Ӧ�Ѵﵽƽ��״̬,B��ȷ;C .��Ӧǰ�����������������,�������������,���������������ܶ�ʼ�ձ��ֲ���,���ж���ӦI�Ѵﵽƽ��״̬,C����;D .��Ӧǰ�����������������,��Ӧǰ�����������Ҳ���ֲ���,����M =m (��)/(��)��֪,�����л�̨����ƽ��Ħ������ʼ�ձ��ֲ������ж���Ӧ�Ѵﵽƽ��״̬,D����;E.c( COS)���ֲ���,�����ж���Ӧ�Ѵﵽƽ��״̬,��ȷ;��������,����ѡBE��
(4) COS(g) +H2O(g)= = H2S(g) +CO2(g)
��ʼ��(mol) 5 5 0 0
�仯��(mol) 5![]() 60%=3 5
60%=3 5![]() 60%3 3 3
60%3 3 3
5min����(mol) 2 2 3 3
�ٷ�Ӧ����ʼ��5 min�ڣ���H2SŨ�ȱ仯��ʾ��ƽ����Ӧ�ٶ�v��H2S����3mol/2L![]() 5min=0.3 mol/(Lmin);
5min=0.3 mol/(Lmin);
�ڸ��¶��µ�ƽ�ⳣ��K��c(H2S)![]() c(CO2)/c(COS )
c(CO2)/c(COS )![]() c(H2O )=1.5
c(H2O )=1.5![]() 1.5/1
1.5/1![]() 1=2.25��
1=2.25��
��A.��Ϊ��Ӧǰ���������,��С�����ݻ�,����ѹǿ,��Ӧ���ʼӿ�,ƽ�ⲻ�ƶ�, COS��ƽ��ת���ʲ������;B .�÷�ӦΪ���ȷ�Ӧ,�����¶�,ƽ������,���ʼӿ�, COS��ƽ��ת���ʼ�С��B����;C.���������,��С������Ũ��,ƽ������, COS��ƽ��ת��������,���ʼ���,����;D.����һ����H2O(g) ,ƽ������,��Ӧ���ʼӿ�, COS��ƽ��ת��������,D��ȷ;��������,����ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��ش����⣺
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
1 | �� | |||||||
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� |
��1���������ڰ뵼����ϵ�Ԫ�������ڱ��е�λ����__________________��
��2���ۡ��ܡ����ԭ�Ӱ뾶��С��___________________����Ԫ�ط����ش𣩡�
��3���ݡ��ޡ��ߵ�����������Ӧ��ˮ���������ǿ����__________������ѧʽ�ش𣩡�
��4���ڡ��ۡ��ܵ���̬�⻯��ȶ�����ǿ����__________�����ṹʽ�ش�
��5���ں͢۰�ԭ����1:2�γɵĻ����������ʽΪ____________���þ��������Ĺ����п�
��������������Ϊ_______________________��
��6���ۺ͢��γɵĻ���������_______________������ӻ�������ۻ���������þ�������________���壨����ӡ��������ӡ�����ԭ�ӡ�����
��7��Ԫ�آݡ��ߵ�����������ˮ���ﻥ�෴Ӧ�Ļ�ѧ����ʽΪ��___________________��
����Ŀ����֪X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ��ʾ(���ֲ�������ȥ)����W��X��������( )

ѡ�� | W | X |
A | ϡ���� | NaAlO2��Һ |
B | ϡ���� | Fe |
C | CO2 | Ca(OH)2��Һ |
D | Cl2 | Fe |
A. A B. B C. C D. D