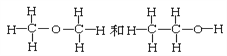
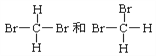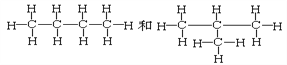ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩœ¬±μΈΣ‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷Θ§«κ≤Έ’’‘ΣΥΊΔΌΘ≠Δα‘Ύ±μ÷–ΒΡΈΜ÷ΟΘ§ΜΊ¥πΈ ΧβΘΚ
Ήε ÷ήΤΎ | ΔώA | ΔρA | ΔσA | ΔτA | ΔθA | ΔωA | ΔςA | 0 |
1 | ΔΌ | |||||||
2 | ΔΎ | Δέ | Δή | |||||
3 | Δί | Δό | ΔΏ | Δύ | Δα |
Θ®1Θ©±μ÷–”Ο”ΎΑκΒΦΧε≤ΡΝœΒΡ‘ΣΥΊ‘Ύ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο «__________________ΓΘ
Θ®2Θ©ΔέΓΔΔήΓΔΔύΒΡ‘≠Ή”ΑκΨΕΉν–Γ «___________________Θ®”Ο‘ΣΥΊΖϊΚ≈ΜΊ¥πΘ©ΓΘ
Θ®3Θ©ΔίΓΔΔόΓΔΔΏΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·ΈοΘ§Φν–‘Ήν«ΩΒΡ «__________Θ®”ΟΜ·―ß ΫΜΊ¥πΘ©ΓΘ
Θ®4Θ©ΔΎΓΔΔέΓΔΔήΒΡΤχΧ§«βΜ·ΈοΘ§Έ»Ε®–‘Ήν«ΩΒΡ «__________Θ®”ΟΫαΙΙ ΫΜΊ¥π
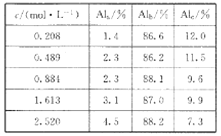
Θ®5Θ©ΔΎΚΆΔέΑ¥‘≠Ή” ΐ1:2–Έ≥…ΒΡΜ·ΚœΈοΒΡΒγΉ” ΫΈΣ____________Θ§ΗΟΨßΧεΤχΜ·ΒΡΙΐ≥Χ÷–ΩΥ
ΖΰΒΡΈΔΝΘΦδΉς”ΟΝΠΈΣ_______________________ΓΘ
Θ®6Θ©ΔέΚΆΔύ–Έ≥…ΒΡΜ·ΚœΈο τ”Ύ_______________Θ®ΧνΓΑάκΉ”Μ·ΚœΈοΓ±ΜρΓΑΙ≤ΦέΜ·ΚœΈοΓ±Θ©Θ§ΗΟΨßΧε τ”Ύ________ΨßΧεΘ®ΧνΓΑάκΉ”Γ±ΓΔΓΑΖ÷Ή”Γ±ΓΔΓΑ‘≠Ή”Γ±Θ©ΓΘ
Θ®7Θ©‘ΣΥΊΔίΓΔΔΏΒΡΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΜΞœύΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ___________________ΓΘ
ΓΨ¥πΑΗΓΩ ΒΎ3÷ήΤΎΓΓIVA Ήε F NaOH HΓΣF ![]() Ζ÷Ή”ΦδΉς”ΟΝΠ Ι≤ΦέΜ·ΚœΈο ‘≠Ή” Al(OH)3+NaOH ===NaAlO2+2H2O
Ζ÷Ή”ΦδΉς”ΟΝΠ Ι≤ΦέΜ·ΚœΈο ‘≠Ή” Al(OH)3+NaOH ===NaAlO2+2H2O
ΓΨΫβΈωΓΩ±ΨΧβΩΦ≤ι‘ΣΥΊ÷ήΤΎ±μΚΆ‘ΣΥΊ÷ήΤΎ¬…ΒΡ”Π”ΟΘ§Θ®1Θ©ΑκΒΦΧε≤ΡΝœ”Π‘ΎΫπ τ”κΖ«Ϋπ τ–‘ΫΜΫγ¥Π―Α’“Θ§ΗυΨί…œ ω‘ΣΥΊ÷ήΤΎ±μΒΡ≤ΩΖ÷ΫαΙΙΘ§ΑκΒΦΧε≤ΡΝœ «ΨßΧεΙηΘ§ΈΜ”ΎΒΎ»ΐ÷ήΤΎΒΎIVAΉεΘΜΘ®2Θ©Ά§÷ήΤΎ¥”Ήσœρ”“‘≠Ή”ΑκΨΕΦθ–ΓΘ§Ά§÷ςΉε¥”…œΒΫœ¬‘≠Ή”ΑκΨΕ‘ω¥σΘ§“ρ¥Υ‘≠Ή”ΑκΨΕ¥σ–ΓΥ≥–ρ «Mg>O>FΘ§Φ¥‘≠Ή”ΑκΨΕΉν–ΓΒΡ «FΘΜΘ®3Θ©Ά§÷ήΤΎ¥”Ήσœρ”“Ϋπ τ–‘Φθ»θΘ§Ϋπ τ–‘‘Ϋ«ΩΘ§ΤδΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΒΡΦν–‘‘Ϋ«ΩΘ§Φ¥NaOH>Mg(OH)2>Al(OH)3Θ§Φν–‘Ήν«ΩΒΡ «NaOHΘΜΘ®4Θ©Ά§÷ήΤΎ¥”Ήσœρ”“Ζ«Ϋπ τ–‘‘ω«ΩΘ§Τδ«βΜ·ΈοΒΡΈ»Ε®–‘‘ω«ΩΘ§“ρ¥Υ«βΜ·ΈοΒΡΈ»Ε®–‘ΘΚHF>H2O>CH4Θ§ΉνΈ»Ε®ΒΡ«βΜ·Έο «HFΘ§ΤδΫαΙΙ ΫΈΣHΘ≠FΘΜΘ®5Θ©Ήι≥…Μ·ΚœΈο «CO2Θ§ΤδΒγΉ” ΫΈΣΘΚ![]() Θ§CO2 τ”ΎΖ÷Ή”ΨßΧεΘ§»έΜ· ±ΩΥΖΰΖ÷Ή”ΦδΉς”ΟΝΠΘΜΘ®6Θ©ΔέΚΆΔύΙΙ≥…ΒΡΜ·ΚœΈο «SiO2Θ§ τ”ΎΙ≤ΦέΜ·ΚœΈοΘ§ΤδΨßΧεΈΣ‘≠Ή”ΨßΧεΘΜΘ®7Θ©Δί «ΡΤ‘ΣΥΊΘ§ΤδΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·Έο «NaOHΘ§ΔΏ «AlΘ§ΤδΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·Έο «Al(OH)3Θ§Al(OH)3±μœ÷ΝΫ–‘Θ§”κΦνΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣAl(OH)3ΘΪNaOH=NaAlO2ΘΪ2H2OΓΘ
Θ§CO2 τ”ΎΖ÷Ή”ΨßΧεΘ§»έΜ· ±ΩΥΖΰΖ÷Ή”ΦδΉς”ΟΝΠΘΜΘ®6Θ©ΔέΚΆΔύΙΙ≥…ΒΡΜ·ΚœΈο «SiO2Θ§ τ”ΎΙ≤ΦέΜ·ΚœΈοΘ§ΤδΨßΧεΈΣ‘≠Ή”ΨßΧεΘΜΘ®7Θ©Δί «ΡΤ‘ΣΥΊΘ§ΤδΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·Έο «NaOHΘ§ΔΏ «AlΘ§ΤδΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·Έο «Al(OH)3Θ§Al(OH)3±μœ÷ΝΫ–‘Θ§”κΦνΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣAl(OH)3ΘΪNaOH=NaAlO2ΘΪ2H2OΓΘ

 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΘ®11Ζ÷Θ©‘Ύ2LΟή±’»ίΤςΡΎΘ§800Γφ ±Ζ¥”ΠΘΚ2NO(g)+O2(g) ![]() 2NO2(g)ΧεœΒ÷–Θ§n(NO)Υφ ±ΦδΒΡ±δΜ·»γ±μΘΚ
2NO2(g)ΧεœΒ÷–Θ§n(NO)Υφ ±ΦδΒΡ±δΜ·»γ±μΘΚ
±Φδ(s) | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)(mol) | 0.020 | 0.01 | 0.008 | 0.007 | 0.007 | 0.007 |
Θ®1Θ©¥οΤΫΚβΒΡ ±Φδ « Θ§άμ”… « ΓΘ
Θ®2Θ©”“ΆΦ÷–±μ ΨNO2ΒΡ±δΜ·ΒΡ«ζœΏ « ΓΘ”ΟO2±μ Ψ¥”0~2 sΡΎΗΟΖ¥”ΠΒΡΤΫΨυΥΌ¬ v= ΓΘ
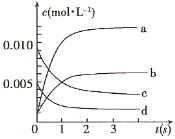
Θ®3Θ©ΡήΥΒΟςΗΟΖ¥”Π“―¥οΒΫΤΫΚβΉ¥Χ§ΒΡ « ΓΘ
aΘ°v(NO2) = 2v(O2) bΘ°»ίΤςΡΎΗςΈο÷ ΒΡ≈®Ε»±Θ≥÷≤Μ±δ
cΘ°vΡφ(NO) = 2v’ΐ(O2) dΘ°¥οΒΫΜ·―ßΤΫΚβ ±Θ§NOΫΪΆξ»ΪΉΣΜ·ΈΣNO2
Θ®4Θ©Ρή‘ω¥σΗΟΖ¥”ΠΒΡΖ¥”ΠΥΌ¬ « ΓΘ
aΘ°ΦΑ ±Ζ÷άκ≥ΐNO2ΤχΧε bΘ° Β±…ΐΗΏΈ¬Ε»
cΘ°‘ω¥σO2ΒΡ≈®Ε» dΘ°―Γ‘ώΗΏ–ߥΏΜ·ΦΝ